INTRODUCTION
The prenatal period is characterized by rapid growth and physiological changes in the fetus. During this period, fetal neurodevelopment involves complex processes of transformation and maturation of the brain and nervous system. Genetic factors influence such processes, which begin early in embryonic development and continue into early adulthood, accentuated by environmental conditions, including smoke exposure. According to the Centers for Disease Control and Prevention (CDC), although the prevalence of maternal smoking during pregnancy in the US has dropped from 7.2% in 2016 to 4.6% in 2021, it remains as high as 13% among minority groups1. While maternal smoking during pregnancy is generally low, around 38% of pregnant women are exposed to secondhand smoke (SHS)2. Prenatal tobacco exposure (PTE) is the exposure of a fetus to tobacco smoke from active maternal smoking or SHS exposure3. This exposes the developing fetus to many hazardous constituents, such as carbon monoxide, nicotine, and carbonyl compounds. Previous research reported a higher risk of low birthweight, premature birth, and poor respiratory outcomes in neonates prenatally exposed to tobacco4. However, the evidence on the effect of PTE on neurodevelopment remains inconclusive. Some studies link maternal smoking and offspring cognitive development, while others found weaker associations after adjusting for confounders like socioeconomic status5. However, this relationship of PTE and offspring neurodevelopment was explored in older children.
The onset of the effects of the prenatal environment is more pronounced in the early years, especially in infants and preschool children. However, early screening for neurodevelopmental abnormalities is challenging due to the lack of efficient tests and limited clinical research in young children6. The objectivity of these assessments has improved in recent years through the development and validation of formal screening tools, such as the Mullen Scale of Early Learning7, Denver II8, and the Pediatric Evaluation of Disability Inventory (PEDI)9. Nevertheless, such tools are not comprehensive enough to address all age groups. These tests are yet to be validated in real-world settings, affecting their reliability in capturing developmental issues in early childhood10. On the other hand, some studies have inadequately evaluated early-life neurodevelopmental outcomes using proxies such as academic performance and IQ tests designed for children of older ages, including the Wechsler Primary and Preschool Scales of Intelligence (WPPSI) and the Peabody Individual Achievement Test (PIAT)11. In contrast, the Bayley Scales of Infant and Toddler Development, 3rd edition (BSID-III) is a widely recognized, validated neurodevelopmental assessment. BSID-III was developed and validated in the US population12 and has several advantages over other measures. Specifically, it can evaluate neurodevelopment in infants as young as one month. Moreover, BSID-III evaluates several domains, including behavioral, cognitive, and motor functions, allowing researchers to investigate the specific aspects affected by the exposures of interest. For these reasons, BSID-III is considered the gold standard for assessing early neurodevelopment13,14.
PTE may be associated with neurodevelopmental delays, which can negatively impact children’s daily activities, family dynamics, academic achievement, productivity, and quality of life. While prior research has focused mainly on genetic, biological, and some environmental factors15, further research, including using larger cohorts and more reliable tools, is necessary to assess PTE’s impact on early childhood developmental trajectories. Our study aims to investigate the temporal association between prenatal PTE and neurodevelopment in infants and toddlers using data from the Environmental Influences on Child Health Outcomes (ECHO)-wide cohort. This program aggregates maternal and child health data from multiple cohorts conducted in the US16.
METHODS
Study population
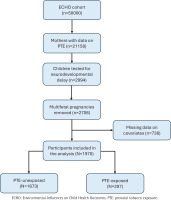
We used data from the ECHO-wide cohort (version 1 dataset), which comprises harmonized and newly collected data from 69 cohorts across the US between October 2018 and August 2021. Access to the data was obtained from Data and Specimen Hub (DASH)16 under a data use agreement between the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) and Qatar University (ECHO-QU-2023-0418). All study participants provided written informed consent for enrollment into ECHO. A detailed protocol containing details on participants recruitment and data collection procedures is described elsewhere17. We included all pregnant women with data on the exposure of interest (prenatal maternal smoking or SHS) and children in whom neurodevelopment was assessed using BSID-III at any time point from infancy to toddlerhood, along with data on maternal age at childbirth and household income level. We excluded offspring of multifetal pregnancies, given the higher risk of adverse fetal and maternal outcomes18. Out of 50000 participants in the ECHO cohort, 21158 mothers had data on PTE. Among these, 2994 children were tested for neurodevelopmental delay. After excluding multifetal pregnancies and participants with missing covariate data, 1970 participants remained in the final analysis. These were categorized as either PTE-exposed (n=297) or PTE-unexposed (n=1673) (Figure 1).
Prenatal tobacco exposure
In the ECHO cohort, PTE was defined as exposure to any form of tobacco smoke, including cigarette smoking and shisha, whether through active maternal smoking during pregnancy or exposure to SHS. Active maternal smoking was assessed using self-reported questionnaires that specifically asked whether the mother used any tobacco or nicotine products during the ECHO index pregnancy. SHS exposure was independently evaluated by determining if anyone in the participant’s household smoked during the pregnancy. We created a binary variable, with ‘yes’ reflecting any prenatal exposure to tobacco and ‘no’ indicating the absence of exposure to any form of smoke.
Neurodevelopmental outcomes
The neurodevelopment of infants and toddlers was assessed using BSID-III, a widely used standardized assessment tool designed to evaluate the developmental functioning of infants and toddlers aged 1–42 months, as recommended by the test developers12. The scale consists of several domains, including cognitive, motor domain, and language skills, which are assessed through direct observation and interaction with the child. The cognitive domain assesses the child’s problem-solving abilities, memory, and attention. The motor domain assesses gross and fine motor skills, and the language domain evaluates expressive and receptive language abilities. Composite scores were obtained for each domain based on the child’s performance during the assessment, with lower scores indicating poorer neurodevelopmental outcomes. The raw scores were then converted into standardized scores to compare the child’s development with age-related norms12. The median standardized score for the US is 100, with a standard deviation of 15. A score between 85–115 is generally considered within the average range. A score ≤85 may indicate developmental delays, suggesting concerns and the need for further evaluation. Infants and toddlers were divided into normal (>85) and delayed (≤85) groups according to the standardized cutoff points reported by Lee et al.19. Based on this cutoff, we generated three independent binary variables that indicated neurodevelopmental delay in the three domains (cognitive, motor, and language). The BSID-III has been validated for use in the US population with Cronbach’s alpha values 0.88–0.9620,21. In our study, the BSID-III achieved a Cronbach’s alpha of 0.82, indicating good internal consistency.
Covariates
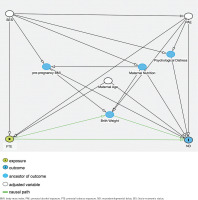
We constructed a directed acyclic graph (DAG) (Figure 2) and included the confounders based on prior evidence, namely, socioeconomic status (SES)22, maternal age23, and prenatal alcohol consumption (PAE). These variables were selected because they are well-documented factors influencing child neurodevelopment. Specifically, maternal age and SES have been consistently linked to various aspects of child development, while maternal alcohol consumption is known to affect neurodevelopmental outcomes. Income level was included as a proxy for SES, categorized based on the household income of US$ 75000, which is the median household income in the US24. Income and education levels are highly correlated in the ECHO data; thus, the education level was excluded from the adjusted model.
Statistical analysis
Median and interquartile range (IQR) or frequencies and percentages were used to describe the participant characteristics. We used two methods to analyze the association between PTE and neurodevelopmental delay. First, we used multivariable linear regression to evaluate the association between PTE and neurodevelopmental delay. This approach was selected to provide a robust estimate of the effect of PTE on mean composite scores of BSID-III while adjusting for potential confounders, namely SES, maternal age, and prenatal alcohol exposure. Our data met all the assumptions of linear regression, including linearity between the predictor and outcome variables, independence of observations, and homoscedasticity, ensuring the validity of our model. Next, we used adjusted logistic regression models to examine the odds of neurodevelopmental delay (defined as a score of ≤85) in PTE-exposed compared to unexposed participants14. The model adjusted for SES, maternal age, and maternal alcohol consumption, representing the minimum adjustment set based on available data. We did not adjust for other covariates as there were missing data or insufficient information regarding these factors in the dataset. We analyzed each domain of the BSID-III independently using STATA version 1825. Results are reported as adjusted odds ratios (AOR) and 95% confidence intervals (95% CI).
Additionally, a mediation analysis was performed to assess the effect of maternal smoking on cognitive performance in children, with birthweight as a mediator. We used the Stata command paramed to estimate the natural direct effect, capturing the effect of smoking independent of birthweight, and the natural indirect effect, representing the portion mediated through birthweight. The marginal total effect reflects the combined direct and indirect effects. Bootstrap-based standard errors were used to compute 95% confidence intervals.
We conducted a power calculation to determine the necessary sample size for this study. To achieve 80% power with a 95% confidence interval, we estimated that at least 201 exposed and 201 unexposed pregnant women would be required to detect an odds ratio of 1.5. This calculation was based on a hypothesized prevalence of neurocognitive developmental delay of approximately 8% in children in the US26. All analyses were conducted in accordance with the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines27.
RESULTS
Table 1 summarizes the characteristics of 1970 mother–child dyads and compares the exposed (297) and the unexposed (1673) groups. The children’s ages span from under six months to over three years. Most participants were of White or Black race, 81.8% and 86.8% in exposed and unexposed groups, respectively. Children born to exposed mothers were less likely to be exclusively breastfed for six months (4.6% vs 13.2%; p=0.004) and had lower median birthweight (1240 vs 2970 g; p<0.001). Mothers in the exposed group were more likely to have some college but no degree (62.5% vs 41.9%; p<0.001), while a greater percentage of women in the unexposed group had a Master’s degree or higher (44.0% vs 27.5%; p<0.001).
Table 1
Demographic characteristics of study sample from ECHO research program, 2018–2021
| Characteristics | Categories | PTE-Unexposed (N=1673) n (%) | PTE-Exposed (N=297) n (%) | p* |
|---|---|---|---|---|
| Age (years) | <0.5 | 45 (2.7) | 13 (4.4) | 0.002 |
| 0.5–1 | 44 (2.6) | 18 (6.1) | ||
| 1–2 | 152 (9.1) | 35 (11.8) | ||
| 2–3 | 879 (52.5) | 150 (50.5) | ||
| ≥3 | 553 (33.1) | 81 (27.3) | ||
| Sex of child (N=1970) | Male | 869 (51.9) | 165 (55.6) | 0.25 |
| Female | 804 (48.1) | 132 (44.4) | ||
| Race (N=1675) | White | 643 (38.7) | 102 (35.8) | <0.001 |
| Black | 799 (48.1) | 131 (46.0) | ||
| Asian | 37 (2.2) | 2 (0.7) | ||
| Hawaiian | 1 (0.1) | 2 (0.7) | ||
| American Indian | 2 (0.1) | 0 (0.0) | ||
| Multiple | 128 (7.7) | 43 (15.1) | ||
| Other | 50 (3.0) | 5 (1.8) | ||
| Child ever breastfed (N=713) | 472 (89.9) | 163 (86.7) | 0.23 | |
| Exclusive breastfeeding for 6 months (N=500) | 46 (13.2) | 7 (4.6) | 0.004 | |
| Mode of delivery (N=1946) | Vaginal | 871 (52.3) | 151 (50.8) | 0.51 |
| Cesarean | 782 (47.0) | 142 (47.8) | ||
| Birthweight (g), median (IQR) | 2970 (1080–3380) | 1240 (840–3105) | <0.001 | |
| Gestational age in weeks, median (IQR) | 38 (28–39) | 29 (26–38) | <0.001 | |
| Preterm (N=1138) | 1029 (61.5) | 109 (36.7) | <0.001 | |
| Maternal education (N=875) | Some college, no degree | 283 (41.9) | 125 (62.5) | <0.001 |
| Bachelor’s | 95 (14.1) | 20 (10.0) | ||
| Master’s or Doctorate | 297 (44.0) | 55 (27.5) | ||
| Household income (US$) (N=1970) | ≥75000 | 372 (22.2) | 20 (6.7) | <0.001 |
| <75000 | 1301 (77.8) | 277 (93.3) | ||
| PAE (N=1970) | Yes | 133 (7.9) | 31 (10.4) | 0.15 |
| Psychological distress (N=769) | 581 (87.6) | 188 (86.6) | ||
| Maternal age at birth (years), median (IQR) | 28 (24–33) | 27 (24–33) | 0.96 | |
| Parity, median (IQR) | 1 (0–1) | 1 (0–2) | 0.17 | |
| Pre-pregnancy BMI, median (IQR) | 27 (23–32.9) | 28.3 (23.6–36.6) | 0.004 | |
| Father’s education (N=701) | Lower than high school | 34 (6.3) | 11 (6.8) | 0.81 |
The multivariable linear regression analysis showed that active maternal smoking was significantly associated with reductions in cognitive and language development scores in children, (B= -4.53; 95% CI: -6.60 – -2.47; p=0.002) and (B= -6.43; 95% CI: -8.87 – -3.99; p<0.0001), respectively. However, no significant association was observed between maternal smoking and motor development (B=0.13; 95% CI: -2.87–3.12; p=0.498). These results suggest that maternal smoking may negatively impact cognitive and language development in children, while motor skills remain unaffected.
Maternal smoking during pregnancy was significantly associated with higher odds of cognitive (AOR=1.84; 95% CI: 1.25, 2.7; p=0.002) and language impairments (AOR=2.04; 95% CI: 1.5–2.8; p<0.0001), but not motor impairments (AOR=1.16; 95% CI: 0.76–1.78; p=0.498). Conversely, secondhand smoke exposure was not significantly associated with cognitive impairments (AOR=1.02; 95% CI: 0.66–1.58; p=0.913) but was linked to reduced odds of language impairments (AOR=0.63; 95% CI: 0.42–0.93; p=0.021), with no significant association for motor delays (AOR=0.85; 95% CI: 0.56–1.3; p=0.461). These findings suggest that maternal smoking poses a greater risk to cognitive and language development compared to secondhand smoke exposure (Table 2).
Table 2
Prenatal tobacco exposure on neurodevelopmental domain composite scores
A causal mediation analysis (Table 3) identified that maternal smoking had both direct and indirect effects on the outcome. The controlled direct effect was estimated to be -1.03 (95% CI: -1.74 – -0.315; p=0.005), showing a significant direct negative connection independent of the mediator (i.e. birthweight). Similarly, the natural direct effect was -0.73 (95% CI: -1.11 – -0.35; p=0.0001), confirming the considerable direct impact of birthweight on the cognitive domain. The natural indirect impact, which captures the mediated pathway, was -0.08 (95% CI: -0.16 – -0.01; p=0.028), indicating a minor but significant indirect effect. The marginal total effect, which considers both direct and indirect pathways, was -0.81 (95% CI: -1.20 – -0.43; p=0.0001), emphasizing the overall detrimental impact of birthweight. The results show that maternal smoking has a negative influence on cognitive function, both directly and indirectly, via its effect on birthweight.
Table 3
Mediation analysis of the effect of maternal smoking on cognitive function, mediated by birthweight
DISCUSSION
We evaluated the association between PTE and neurodevelopmental delay in infants and toddlers using 1970 mother–child dyads from the ECHO-wide cohort. Our findings demonstrate that active maternal smoking during pregnancy is strongly associated with delays in all three domains. At the same time, SHS exposure did not notably increase the odds of delay in these domains.
Maternal smoking during pregnancy was associated with a significant increase in the odds of cognitive and language delays. These findings align with earlier studies that reported maternal smoking negatively impacts child neurodevelopment, with AORs ranging from 1.4 to 2.47-19. The association was present for motor delays but not as strong as those observed for other neurodevelopmental domains, suggesting a weaker or less consistent impact of PTE on motor development.
In contrast, SHS exposure showed no increase in the odds of cognitive, language, or motor delays. Our results are consistent with the findings of Batty et al.11, who found no difference in IQ scores between those exposed and unexposed to SHS after adjusting for maternal education or IQ11. In contrast, other studies examining the effects of SHS on neurodevelopmental outcomes in children aged 5–18 years showed a weaker association between PTE and neurodevelopmental delay after adjusting for birthweight, parental behavior, and postnatal smoking11,28,29. Our findings may be limited by the method used to measure SHS exposure in ECHO (i.e. a single yes/no question), which solely assessed whether the mother lived with a smoker during pregnancy. SHS exposure can arise from various sources, including the residential area, workplace, or other social environments30, making a single question insufficient to capture the full extent of exposure.
Our research adds to the body of evidence by focusing on infants and toddlers aged 1–42 months, whereby prenatal influences on neurodevelopment are more prominently manifested. Our results also align with studies that used more objective measures to assess maternal smoking. For instance, Polanska et al.31 found an association between maternal cotinine levels and motor, cognitive, and language delay in children aged 24 months31. Despite the potential stigma associated with reporting tobacco exposure during pregnancy, self-reported data from the ECHO-wide cohort captured over 15% of PTE-exposed mothers. This large cohort enables a precise evaluation of the effects of PTE on offspring neurodevelopment. Although our study did not employ quantified cotinine levels, as used by Polanska et al.31, it offers reliable estimates with broad generalizability to the US population.
Our assessment of the mediation effect of birthweight on the association between PTE and neurodevelopmental delay revealed that maternal smoking negatively impacts the child’s cognitive function, partly through the effect of low birthweight. This highlights the dual pathway by which maternal smoking harms offspring, emphasizing the need to address both direct and birthweight-mediated effects in interventions targeting prenatal health. The effect of maternal smoking during pregnancy on low birthweight has been well-documented in the literature32. Consistent with our findings, previous research has also identified birthweight as a mediator in the relationship between maternal smoking and various childhood disorders, including elevated blood pressure and behavioral disruptions33. However, one study found no mediation effect of birthweight on the association between maternal smoking and lowered IQ in offspring34.
The biological mechanisms underpinning these findings highlight the potential effects of the harmful components of tobacco smoke, such as nicotine and carbon monoxide, on fetal brain development. Nicotine crosses the placental barrier and may disrupt the nicotinic receptors in the fetal central nervous system, impairing neural development35. Additionally, nicotine exposure alters neurotransmitter function and reduces the nutritional supply to the fetus by inducing anorexic effects. Carbon monoxide could further restrict oxygen delivery by forming carboxyhemoglobin and constricting blood vessels, reducing blood flow to the fetal central nervous system36. These mechanisms may account for the observed cognitive and language delays associated with maternal smoking.
Strengths and limitations
Our study has several strengths. By using a large cohort of 1970 mother–child dyads, we were able to provide robust estimates of the effects of maternal smoking on neurodevelopment. We used BSID-III as it is the gold standard for assessing neurodevelopment in infants and toddlers. Additionally, the inclusion of both direct and indirect tobacco exposure allows for a comprehensive evaluation of the impact of active maternal smoking and SHS exposure on child development. The retrospective cohort design also establishes temporality, ensuring that maternal smoking occurred prior to neurodevelopmental outcomes, which is critical in understanding causal relationships. However, there are some limitations to consider in this study. Although the data were collected prospectively, reporting bias may still arise due to the sensitive nature of the questions, potentially leading to underreporting of maternal smoking during pregnancy. Moreover, the ECHO dataset did not have information on the use of newer tobacco products, such as e-cigarettes, which may contribute to prenatal tobacco exposure and could influence neurodevelopmental outcomes. Additionally, smoking status and secondhand smoke exposure were recorded as yes/no responses, preventing us from assessing a dose-response relationship. Residual confounding may also be present despite adjusting for key covariates. Furthermore, the socio-emotional and adaptive domains of the BSID-III were not included in this study, limiting a more comprehensive assessment of neurodevelopment. Lastly, as the data were drawn from the US population, the findings may not be fully generalizable to other countries with different smoking patterns, environmental exposures, or healthcare systems.
Future research
Future research can benefit from utilizing biomarkers that quantify tobacco use in addition to self-reported data to avoid potential measurement errors. Assessing PTE at multiple time points during pregnancy could also provide an opportunity to assess the true extent (length and level) of how exposure affects offspring neurodevelopment. The longitudinal approach can also prove beneficial in identifying the cumulative effects of both active and SHS during pregnancy.
CONCLUSIONS
Our study suggests that prenatal exposure to tobacco may increase the odds of cognitive and language neurodevelopmental delays in early childhood, though it appears less likely to affect motor impairment. Our findings suggest that the deleterious effect of PTE on neurodevelopment can begin as early as the in utero period. Future studies should explore interventions to address PTE and reduce the risk of neurodevelopmental delays in offspring.