INTRODUCTION
Tobacco use remains the single most modifiable cause of adverse pregnancy outcomes, yet, more than 50% of smokers who become pregnant continue to smoke1,2. In addition, for social acceptability reasons, many pregnant women underreport or misrepresent their smoking status3-5. The adverse effects of nicotine to the fetus are multiple and well-described, specifically on growth, lung development, asthma, postnatal infections and sudden infant death syndrome (SIDS)3. Numerous reports have also established that maternal tobacco smoking during pregnancy is associated with increased risks for adverse maternal conditions (e.g. premature rupture of membranes, abruptio placenta, and placenta previa) and adverse pregnancy outcomes (e.g. neonatal mortality and stillbirth, preterm delivery, and SIDS)6-8. It is crucial to be able to accurately quantify the burden of tobacco exposure on both the mother and fetus to have efficacious measures in intervention studies.
Numerous maternal and fetal biomarkers have been used to quantify both direct and indirect exposure to nicotine and tobacco exposure, including exhaled carbon monoxide, nicotine or cotinine in urine, saliva, and serum, and urinary 4-(Methylnitrosamino)-1-(3-pyridyl)-1-butanol (NNAL), with most reflecting short-term exposure9-11. NNAL has a longer half-life but is not present in nicotine replacement products or e-cigarettes making it less useful for those products11. Several studies have reported correlations between the hair nicotine levels of mother–infant dyads exposed to secondhand smoke, which was able to reflect a longer duration of exposure (over a period of several months) compared to the other biomarkers12-15. Toenail nicotine levels were also reported to capture the longer term overall burden of tobacco smoke exposure in adults5,16, while newborn nails have been demonstrated to reflect drug exposure to substances such as caffeine, nicotine, cocaine, benzoylecgonine, morphine, and methadone, during pregnancy17. In our randomized placebo-controlled trial of pregnant women who smoke, we reported urine cotinine as our biomarker for maternal smoking status, but to our knowledge, no other study has collected maternal and infant hair and nail samples at delivery alongside a prospective collection of serial maternal smoking questionnaires and urine cotinine levels for the duration of the pregnancy, to determine if nicotine in infant hair and nail could be reliable biomarkers of in-utero exposure to tobacco smoke18. Our study aims to demonstrate that infant hair and nail nicotine levels collected shortly after birth correlate with maternal hair and nail nicotine levels and reflect maternal cigarette/nicotine exposure during pregnancy.
METHODS
This is a descriptive and exploratory study performed within a larger, randomized, double-blind, placebo-controlled trial of pregnant women who were unable to quit smoking, and were randomized to receive vitamin C (500 mg/day) versus placebo prior to 22 weeks gestation with the primary outcome being pulmonary function tests (PFTs) in the newborns after delivery (NCT00632476). A group of pregnant non-smokers and their newborns was studied as a reference group. The protocol was approved by the Institutional Review Boards of the participating institutions, and each pregnant woman provided written informed consent. Study participants were recruited from prenatal clinics at: Oregon Health & Science University (OHSU) and Providence Maternal Care Clinic in Portland, Oregon; and Vancouver Clinic and PeaceHealth Southwest Medical in Vancouver, Washington; who were aged ≥15 years, current smokers (≥1 cigarette/day), with singleton gestation, randomization at ≤22 weeks’ gestation age (GA) by last menstrual period (LMP), and had declined smoking cessation attempts as discussed by their physician and study personnel. Exclusion criteria were multiple gestation, major fetal anomalies, current illicit drug or alcohol abuse, continuous daily high dose vitamin C since LMP, insulin dependent diabetic, or history of kidney stones. A group of pregnant non-smokers, aged ≥15 years, with a singleton gestation ≤22 weeks’ GA was recruited as our reference group18. The parent study demonstrated that the offspring of pregnant smokers randomized to Vitamin C had significantly improved PFTs at delivery and had a significant decrease in the incidence of wheeze through one year of age18.
A detailed smoking history using questions from a standardized respiratory questionnaire published by the American Thoracic Society for epidemiologic research19 was collected at each visit to determine the amount of cigarettes smoked and cigarette exposure, along with other important demographic characteristics. The parent study was started before widespread use of smokeless tobacco products, so these products were not assessed. During subsequent visits, up until the time of delivery, brief smoking cessation counseling (<10 minutes) was performed based on American College of Obstetricians and Gynecologists guidelines20, and an interval maternal smoking questionnaire was administered to determine the extent of smoking and/or exposure since the last visit. Urine cotinine was obtained and measured at 24, 30, and 34 weeks of gestation to confirm tobacco smoke exposure reported in the questionnaires21.
Samples of maternal hair and nail were obtained at the time of delivery, and neonatal hair was obtained within 48 hours after delivery. Because of innate difficulties in obtaining infant nail samples (inadequate amount for 1 collection), mothers were also instructed to collect infant nail clippings for approximately the first 2–3 months of life and return them for nicotine analysis. Hair and nail samples were stored in small sealed plastic bags, and sent to the biochemical laboratory at Wellington Hospital (New Zealand), with nicotine extracted and measured (ng/ mg) using high-performance liquid chromatography (HPLC) with electrochemical detection method as described and validated by Mahoney & Al-Delaimy22 and by Al-Delaimy & Mahoney16. As described previously, hair and nail samples were washed with dichloromethane to remove environmental nicotine then digested with 1M NaOH prior to HPLC separation16,22. Laboratory staff were blinded to the smoking status of the maternal/neonatal samples. To reduce random error, samples were run in duplicate when there were enough samples (69% of hair samples, and 24% of nail samples).
Specimen collection
Hair: About 10–20 strands of hair were cut as closely as possible to the scalp at the occipital area for both maternal and neonatal samples, with the proximal end marked and the sample tied with a string and stored at room temperature in a sealed paper envelope.
Nails: Fingernails were clipped and stored in a small static-free plastic zip bag at room temperature. At least 20 mg of nail samples were collected for every participant. For the newborn infant, to approximate the minimum amount of nail samples needed for analysis, fingernail and toenail clippings were collected for the first 3 months of life by the mother and placed in a small static-free plastic zip bag. This was either mailed back to the study team in a sealed, self-addressed pre-stamped envelope that was provided to them or returned in person during a postnatal visit.
Maternal urine was collected at enrollment and subsequent study visits (if available), and cotinine levels were analyzed using a chemiluminescence based immunoassay on an Immulite auto-analyzer (Siemens Health Care Diagnostics)21
Standard maternal and neonatal data were collected prospectively, and included medical, social and delivery history, including co-morbidities, medications, tobacco use, and alcohol use.
Maternal information about hair color, hair treatments and use of hair products were documented, as well as use of nail polish, manicures, or pedicures.
Statistical analysis
Descriptive statistics were used to characterize maternal and neonatal demographics. We used the t-test to look at the difference in mother–baby hair and nail nicotine levels according to smoking status (smokers vs non-smokers). Raw hair and nail nicotine levels were log transformed using the natural logarithm as the base due to their non-normal distribution. The correlations between the variables and outcomes were performed by the Pearson method, with computed p-values for their significance. All p values are 2-sided, and significance was set at p<0.05. Statistical analyses were conducted using R version 4.0.3.
RESULTS
Forty-six dyads (46) within the cohort had successful completion of all four collection samples (34 pregnant smokers and 12 pregnant non-smokers) for hair and nails. This is an exploratory study started after the main study had already been initiated, so only a subgroup of participants was able to provide all four samples of hair and nails. Table 1 shows the maternal and infant demographics of our study population, with the two groups showing no significant difference in maternal age, mode of delivery, mean gestational age of infant at the time of delivery, and mean birthweight. There is a higher percentage of Medicaid insured mothers who smoke compared to non-smokers. Table 2 summarizes our primary outcome of nicotine levels in hair and nails of the two groups of mother–infant dyads within our cohort. The median hair nicotine level for mothers who smoke was significantly higher than that of the non-smokers (1.015 vs 0.037 ng/mg, p<0.05), while the hair nicotine level of infants born to mothers who smoke was also significantly higher than the infants of the non-smokers (0.445 vs 0.080 ng/mg, p<0.01). Similarly, we found that the median nail nicotine levels for mothers who smoke were significantly higher than the non-smokers (2.130 vs 0.056 ng/mg, p<0.01) and the median nail nicotine levels of their respective infants reflected the same pattern (0.594 vs 0.132 ng/mg, p<0.05).
Table 1
Maternal and infant characteristics of the study cohort (N=46)
| Characteristics | Non-smoker Median or n (%) | Percentiles* (Q1; Q3) | Smoker Median or n (%) | Percentiles* (Q1; Q3) |
|---|---|---|---|---|
| Total, n | 12 | 34 | ||
| Maternal | ||||
| Cigarettes/day | 0 | 0 | 7.29 | (2; 12) |
| Maternal age (years) | 26.25 | (19.75; 30.25) | 25.85 | (20; 28.75) |
| Gravida | 2 | (1; 2) | 3.09 | (1; 5) |
| Parity | 0.58 | (0; 1) | 1.323 | (0; 2) |
| Insurance (Medicaid vs gov’t insurance) | 6 (50.0) | 32 (94.0) | ||
| Maternal asthma | 2 (17.0) | 7 (20.0) | ||
| Race | ||||
| White | 10 (83.3) | 27 (79.4) | ||
| Black | 1 (8.3) | 6 (17.6) | ||
| American Indian | 0 | 1 (3.0) | ||
| Asian | 1 (8.3) | 0 | ||
| Maternal education level | ||||
| Some grade school | 1 (8.3) | 6 (17.6) | ||
| Some high school | 6 (50.0) | 21 (61.8) | ||
| Some college | 2 (16.7) | 7 (20.6) | ||
| Some postgraduate | 3 (25.0) | 0 | ||
| Other maternal and infant | ||||
| Maternal hair treated/dyed/bleached | 1 (8.0) | 6 (18.0) | ||
| Maternal nails polished/manicure/pedicure | 3 (25.0) | 6 (18.0) | ||
| Delivery mode (cesarean section) | 3 (25.0) | 8 (24.0) | ||
| Infant GA (weeks) | 38.3 | (37.5; 39.9) | 38.9 | (38.1; 40) |
| Birthweight (g) | 3175 | (2765; 3739) | 3326 | (2956; 3536) |
| Sex | ||||
| Male | 4 (33.0) | 18 (53.0) | ||
| Female | 8 (67.0) | 16 (47.0) |
Table 2
Nicotine levels in hair and nails of pregnant smokers and non-smokers, and of their offspring, collected at delivery (N=46)
| Biomarker | Non-smoker and infant dyad Median | Percentiles* (Q1; Q3) | Smoker and infant dyad Median | Percentiles* (Q1; Q3) | p |
|---|---|---|---|---|---|
| Total, n | 12 | 34 | |||
| Cigarettes/day | 0 | 7 | (2; 12) | ||
| Urine cotinine (ng/mL) | 10 | (10; 14.42) | 3019 | (861; 6712) | |
| Maternal hair nicotine (ng/mg) | 0.037 | (0.01; 0.09) | 1.015 | (0.56; 2.76) | <0.05 |
| Infant hair nicotine (ng/mg) | 0.080 | (0.03; 0.16) | 0.445 | (0.19; 0.69) | <0.01 |
| Maternal nail nicotine (ng/mg) | 0.056 | (0.02; 0.08) | 2.130 | (0.48; 4.14) | <0.01 |
| Infant nail nicotine (ng/mg) | 0.133 | (0.03; 0.37) | 0.594 | (0.24; 1.17) | <0.05 |
* Q1=25th, Q3=75th percentile. Urine cotinine based on 1 to 3 occasions for each participant (2 missing non-smokers, 7 missing from smokers). Samples collected from subjects recruited at: Oregon Health & Science University (OHSU) and Providence Maternal Care Clinic in Portland, Oregon; and Vancouver Clinic and PeaceHealth Southwest Medical in Vancouver, Washington.
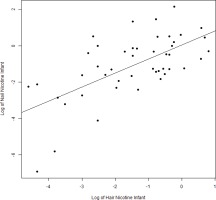
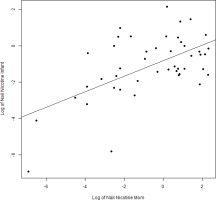
We found expected correlations between maternal hair and nail nicotine levels with cigarettes per day (r=0.60 and r=0.53; p<0.001, respectively), as well as maternal hair and nail nicotine levels with average urine cotinine during the pregnancy (r=0.82 and r=0.6; p<0.001). There are strong correlations between maternal hair and maternal nail nicotine (r=0.64, p<0.001) as well as between infant hair and infant nail nicotine levels (r=0.64; p<0.001) (Figure 1). The correlations between maternal and infant hair nicotine levels (r=0.61, p<0.001) as well as maternal and infant nail nicotine levels (r=0.58, p<0.001) (Figure 2), were also significant.
DISCUSSION
The levels of nicotine, its major metabolite cotinine, and tobacco-specific nitrosamines have been used as biomarkers for the quantitative assessment of tobacco smoke exposure in numerous studies10,12,14,23,24. Cotinine, a major metabolite of nicotine, has been extensively measured in urine and plasma as a biomarker of firsthand and secondhand smoke exposure. However, these measurements can only reflect the last few days of tobacco smoke exposure at best, despite cotinine having a longer half-life than nicotine in these samples. Hair nicotine have been shown to capture chronic exposure in adults and children, while toenail nicotine has more recently been demonstrated to do the same in adults. The advantages of hair and nail nicotine levels over plasma and urine in providing better information on long-term or chronic exposure, and the non-invasive nature of specimen collection make them ideal biomarkers of exposure for infants and children. These samples can also be stored and analyzed years later, without requiring sophisticated storage methodologies or technology.
Analysis of maternal and neonatal hair as a biological marker for nicotine exposure during pregnancy has been studied by Klein and Koren24 and Pichini et al.25. In the study by Klein and Koren24, they found a significant correlation between maternal and neonatal hair nicotine levels of 94 dyads, with maternal levels being significantly higher than newborn levels across 3 group classifications (active smokers, passive smokers, non-smokers). In the study by Pichini et al.25, nicotine could not be quantified in 30.7% of newborn hair samples, and they found no correlation between maternal hair nicotine and newborn hair nicotine levels. In their study, nicotine concentration in hair of newborns from non-exposed non-smokers and exposed non-smokers were also similar, a contrast to the observation by Klein and Koren24. The two studies used different assay methodologies, with the former using radioimmunoassay technique, while the latter used gas chromatography24,25. Our study used reverse-phase high performance liquid chromatography with electrochemical detection, a methodology developed and validated by Mahoney & Al-Delaimy22, Al-Delaimy & Mahoney16, and by Al-Delaimy & Willett5, and despite using a different assay and result values, our study findings are more consistent with the findings of Klein and Koren24, where we found a strong correlation between maternal and newborn hair nicotine levels, demonstrating that newborn hair nicotine can be a valuable predictor of fetal exposure to cigarette smoke. An unexpected observation in our dataset was that in our non-smoking group, the median newborn hair and nail nicotine levels appeared to be higher than the maternal levels. However, these levels were still significantly lower than the respective levels in the babies of smokers. This could be a marker of secondhand smoke exposure that was not captured by the respiratory questionnaires administered during pregnancy.
It is also interesting to note that we found that the levels of nicotine detected in the nails were higher than those noted in the hair among both our infant and maternal smoking and non-smoking samples, in contrast to the findings of Al-Delaimy & Mahoney16 and Al-Delaimy & Willett5, whose studies have shown lower levels of nicotine in nails than in hair in adults. Human nails have been used to monitor excessive exposure to elements for many years, and there is an abundance of literature that has reported the use of nail analysis in postmortem detection of drugs of abuse, drug testing in the workplace, and drug screening to detect prenatal exposure, even though further studies are needed for correct interpretation of the data obtained26,27. Palmeri et al.26, have shown that drugs are incorporated into nails by a double mechanism: 1) deposition into the root of the growing nail via the blood flow in the nail matrix; and 2) incorporation via the nail bed during growth from the lunula to the beginning of the free margin. Unlike hair, nails are not influenced by melanin content, which is a potential confounder for nicotine deposition28-30. We therefore speculate that our results may reflect the fact that the deposition of nicotine in the nails is less affected by acute and short-term environmental changes applied to the human body compared to hair, which can be altered or influenced by race/ethnicity, type of hair, hair color, or treatments done to the hair (bleaching, straightening, permanent, etc.)31. Our findings that newborn nail nicotine levels were higher than newborn hair nicotine levels could represent the susceptibility of nails to nicotine deposition prenatally, as the formation of nail beds occur earlier in the fetus (at around 8–10 weeks’ gestation), which do not undergo shedding in-utero compared to hair, and are shed into the amniotic fluid prior to delivery32.
This is the first study to quantify and demonstrate that newborn nail nicotine levels can be used to assess in-utero tobacco smoke exposure. Babies born to mothers that were unable to quit smoking during pregnancy had significantly higher levels of nail nicotine levels than those born to non-smokers. In addition, we demonstrated that the nail nicotine levels had a strong correlation with other biomarkers of nicotine exposure including infant hair nicotine levels and maternal hair and nail nicotine levels at delivery. Baby nail nicotine levels also had a significant correlation (r=0.62; p<0.001) with maternal urine cotinine levels obtained during pregnancy, a more standard measure of nicotine exposure, while they had a moderate correlation (r=0.44; p<0.001) with maternal reported cigarettes per day during pregnancy. To our knowledge, no other study had investigated nail nicotine in newborns in relation to maternal nail nicotine levels and smoking status during pregnancy. Al-Delaimy and Willett5,33 demonstrated that toenail nicotine levels captured the tobacco smoke exposure in a large cohort of women that participated in the Nurses’ Health Study and were a strong predictor of lung cancer in a large male cohort within the Health Professionals Follow-up Study. Mari et al.22 reported that nicotine was detected in newborn nails, but did not have maternal data to correlate with in-utero exposure and nicotine levels were not quantified.
Strengths and limitations
The strength of our study lies in the prospective collection of multiple biomarkers (maternal urine cotinine, maternal and newborn hair and nail nicotine) accompanied by serial respiratory questionnaires, collected during pregnancy and postnatally from the same mother–infant dyads. Nicotine extraction and measurements were also performed at a well-established and published laboratory with rigorous testing procedures. Nicotine also has the advantage of being the primary bioactive compound and present in all tobacco products, e-cigarette and nicotine replacement products. A limitation would be a possible underestimation of nicotine exposure from self-reported questionnaires. Pregnant women when asked to provide information on smoking habits, often underreport actual behaviors because of social pressures and possible feelings of guilt, if they think it is unhealthy for the baby and themselves. Also, the non-smokers in the cohort included former smokers who had quit prior to pregnancy and may have continued to be passively exposed to tobacco smoke but not captured in our study questionnaire. We also have a relatively small sample size due to difficulty obtaining hair and nail samples from the newborn infant. We found that mothers would consent to the taking of their own samples but often hesitated in agreeing for hair to be cut from their newborn for aesthetic or cultural reasons. We did not analyze maternal urine for tobacco-specific nitrosamines, which have a relatively long half-life and are also specific to conventional tobacco products as they are not present in e-cigarettes and nicotine-replacement products10. However, despite these limitations, our study shows that newborn nail nicotine levels correlate well with maternal nail nicotine levels, with levels significantly higher for the smoking group compared to the non-smokers, and newborn levels showing a strong correlation with maternal levels.
CONCLUSIONS
Our study shows that both infant hair and nail nicotine levels are valid biomarkers of intrauterine tobacco smoke exposure, and can be used to identify prenatal smoke exposure, correlating well with the level of maternal nicotine exposure. Measuring nail nicotine levels in newborns provides an advantage over hair nicotine levels in situations where newborn hair is unavailable or inadequate, and may better reflect prenatal smoke exposure due to the earlier development of nail beds compared to hair follicles in the fetus. This finding has many possible applications in clinical and research settings to determine causes of respiratory illnesses of the newborn, low birthweight, or longer term childhood illnesses.