INTRODUCTION
Since December 2019, there has been an outbreak of pneumonia of unknown etiology that was first reported in Wuhan, Hubei Province, China. Following the outbreak, a novel coronavirus SARS-CoV-2 disease, COVID-19, was identified by the World Health Organization (WHO) as the causative virus for the pandemic in China and other parts of the world with more than 30 million cases of infection and 0.9 million deaths globally1. In addition, COVID-19 pandemic caused poor mental health and quality of life, as reported. This pandemic is seen to be far from over and there is a continuing resurgence in many countries. The COVID-19 pandemic has caused panic and anxiety because of the increasing number of COVID-19 cases worldwide2,3. Furthermore, COVID-19 has had a significant global economic impact and a huge burden on healthcare resources4.
Smoking has been assumed to be associated with adverse disease prognosis, as extensive evidence has highlighted the negative impact of tobacco use on lung health. It is also found to be detrimental to the immune system and its responsiveness to infections, making smokers more vulnerable to infectious diseases5. Smoking increases the risk and severity of pulmonary infections because of damage to upper airways and a decrease in pulmonary immune function6. It still remains controversial, however, if smoking results in severe symptoms and death among COVID-19 patients. Some previous studies reported a significant association between current smoking, former versus never smoking with COVID-19 negative outcomes7–10. The differences between risk of severity and death between former and never smoker COVID-19 patients have not been shown11–13. Because of small sample sizes included in these previous studies and differing definitions of disease severity, existing systematic reviews and meta-analyses found limited evidence suggesting that the risk of COVID-19 infection maybe lower among smokers compared to non-smokers, albeit from highly heterogeneous studies14–18.
There were a number of factors related to the severity of COVID-19 and the mortality rate, including: older age (>65 years), comorbidities (e.g. hypertension, diabetes), organ dysfunction, lymphopenia, high cytokines, and weak immune responses19–22. Especially, older age was associated with a dramatically higher risk of severe COVID-19. For example, the case fatality rate in three databases exceeded 1% around the age of 50–55 years, but was 10% above 80–85 years (≥70 years in Italy)23. Males aged >65 years, and smoking patients, face greater risk of developing a severe or critical condition19. A previous meta-analysis showed that all age groups had significantly higher mortality compared to their immediately younger age group, with the largest increase in mortality risk observed in patients with ages 60–69 compared to 50–59 years24. This fact could be influenced by both the aging process and the high prevalence in frailty and comorbidities among the older people, which contribute to a decrease in their functional capacity.
Given the unclear evidence about smoking in COVID-19 infected patients aged ≤65 years, we conducted a comprehensive SR/MA to determine the association between smoking and disease severity in COVID-19 infected patients by including all eligible studies. Systematic searching of databases for available evidence and careful definition of disease severity was performed for a rigorous summary of the conclusions.
METHODS
Protocol and registration
The systematic review and meta-analysis were performed in line with the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) statement25. This research was registered with PROSPERO (Registration Number CRD42020186638). Patients and the public were not involved in this study. The systematic review and meta-analysis are exempt from ethics approval because data were collected and synthesized from previous studies. The patient data are anonymized and available in the public domain. The authors followed applicable EQUATOR Network (https://www.equator-network.org) guidelines during the conduct of research project.
Data sources and search strategy
To find relevant studies, scientific databases including Embase, PubMed, Science Direct, Google Scholar and Cochrane Library databases were systematically searched from their inception to 12 December 2020. Medical Subject Headings (MeSH) were used whenever applicable. Bibliographic lists of related articles were explored. The search strategy was carried out with the following keywords: [tobacco OR smok*] AND [covid OR coronavirus OR sars cov*] with slight adjustments depending on the database. There was no study design and language restriction. Additionally, extra searches were performed in the reference lists of included studies to avoid missing any article (Supplementary file Table S1).
Study selection
All relevant articles that reported clinical characteristics and epidemiological information on smoking among COVID-19 infected patients were included in the analysis. All articles with any design (randomized controlled trials and observational studies) were included. Animal studies, reviews, commentaries, editorials, expert opinions, letters, conference meeting abstracts, case reports, case series, systematic reviews and meta-analyses were excluded. Studies with the same participants that did not include effect estimates or had insufficient data to measure effect estimates were also eliminated. Articles with explicit involvement with the tobacco industry were excluded.
Outcomes measures
The primary outcome was disease severity among COVID-19 patients with a history of smoking. The secondary outcome was death among COVID-19 patients with a history of smoking. The term ‘disease severity’ includes clinical presentations based on physical examinations and laboratory results, and other medical records, as diagnosed and described by physicians.
Disease severity was defined by any of the following criteria.
Patients who required ICU care26.
Severe case as defined by the American Thoracic Society guidelines for community-acquired pneumonia22.
Severe stage, if any of the following criteria existed:
a) shortness of breath, respiratory rate ≥30 times/min; b) oxygen saturation <93% in resting state; c) PaO2/FiO2 ≤300 mmHg. CT imaging showed significant lesion progression >50% within 24 to 48 h; d) respiratory failure requiring mechanical ventilation; e) shock; and f) complications with other organ failure requiring ICU care27.
Severe cases were patients needed supplemental oxygen therapy28.
Severe cases or patients with Acute Respiratory Distress Syndrome (ARDS) having PaO2/FiO2 ≤300 mmHg29.
Severe or critical patients as defined by the General Office of National Health Commission of China, version 5 (2020)30.
In cases where smoking status did not specify type of smoking, it was taken to be current smoking.
Data extraction and quality assessment
Two investigators (AU and SK) independently screened each title, abstract and full-text article for potentially eligible studies. Discrepancies were resolved by discussions with a third investigator (SS). All extracted data were independently reviewed by two investigators (AU and SK). The following information was extracted from each study: setting, region, design, sample size, demographic characteristics of participants (age, sex), details of intervention/exposure (smoking status: current or former smoker), and details of outcomes (disease severity: severe or critical vs non-severe; death), and number of COVID-19 patients. The quality of individual studies was appraised independently using the Newcastle–Ottawa Scale (NOS)31. The NOS assigns a maximum of 9 points, with studies having a total score of ≥7 defined as high quality.
Statistical analysis
We computed odds ratio (OR) and 95% confidence interval (CI) for each study using the number of smokers (former or current) and never smoker with pre-specified outcomes (severity and death). The pool effects were combined using random-effect model. Heterogeneity was investigated using Cochran’s Q statistic and I2. Cochran’s Q statistic with an alpha value of 0.10 was chosen to designate heterogeneity amongst trials for each analysis. Heterogeneity level was assigned as: I2 >75%, 25–75%, and <25% to indicate high, moderate, and low level, respectively31. In the case where heterogeneity existed, an attempt to explore possible sources of heterogeneity was made. Publication bias was assessed using Begg’s test, Egger’s test, and funnel plot32–34. A p<0.05 in publication bias tests was suggestive of publication bias. When publication bias was found, the trim-and-fill method was used35.
Sensitivity and subgroup analysis
To appraise the robustness of our analysis, the sensitivity analysis for unmeasured confounding was used. Subgroup analyses were conducted by age differences between groups, current and former exposure to smoking, and quality of the studies. Meta-regression analysis was performed using random-effects meta-regression, metareg command in STATA software36, adjusting for study characteristics (covariates) on pooled outcome. The following potential moderator variables: age (>65 years), hypertension and diabetes mellitus were included for meta-regression analysis.
RESULTS
Search results and characteristics of studies included
In the initial search, 1248 articles were retrieved from all databases. Of these, 159 were eliminated that were found to be duplicates. All articles were screened using the title and abstract. After evaluating the abstracts, 937 studies were excluded due to their data being irrelevant to our objective. After evaluating the full text, a total of 40 studies with 369287 COVID-19 infected patients were included in the meta-analysis (Figure 1). The important characteristics and outcomes of the included articles were collated (Table 1). Of 40 articles, 19 were conducted in China21,22,27,29,30,37–50, one in Kuwait26, one in Korea28, one in Mexico51, one in Japan52, two in Spain53,54, three in Italy55–57, and twelve in the USA33,58–68. Most articles were retrospective studies. The mean age of the patients in the included studies was 54.10 years. Nineteen studies defined outcomes as disease severity22,27–30,37–39,42,43,46–50,58,60,62,63. Seventeen studies defined outcomes as death21,33,40,44,45,51–57,59,65–68. Four studies used both disease severity and death26,41,61,64. All studies defined smoking status as current smoker. Eleven studies included former smokers and current smokers22,33,43,44,47,57,58,61–63,67.
Quality assessment
Newcastle–Ottawa scale was used to assess the methodological quality of the 40 studies. Results showed 12 studies receiving ≥7 stars26,44,49,55–57,59–62,64,68, and the remaining studies receiving <7 stars (Supplementary Table S2).
Synthesis of results
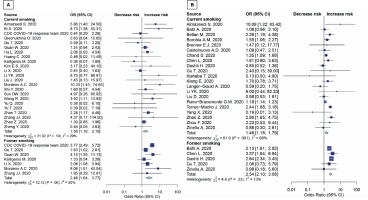
The results in younger patients (≤65 years) showed that both current smoking and former smoking significantly increase the risk of disease severity (OR=1.58; 95% CI: 1.16–2.15, p=0.004; and OR=2.48; 95% CI: 1.64–3.77, p<0.001; respectively) (Figure 2A). Moreover, both current smoking and former smoking also significantly increase the mortality risk in COVID-19 patients (OR=1.35; 95% CI: 1.12–1.62, p=0.002; and OR=2.58; 95% CI: 2.15–3.09; p<0.001; respectively) with moderate appearance of heterogeneity (Figure 2B).
Sensitivity and subgroup analyses
These analyses were conducted for patients >65 years. Results showed that both current smoking and former smoking significantly increase the risk of death (OR=1.46; 95% CI: 1.18–1.79, p=0.002; and OR=2.54; 95% CI: 2.10–3.08, p<0.001; respectively) (Figure 3B). There were no studies with patients aged >65 years in severity outcome (Figure 3A). The sensitivity analysis for unmeasured confounding for death outcome remained substantial (OR=1.38; 95% CI: 1.12–1.71, p=0.003). The subgroup results were consistent with the main study results mentioned above. Details are shown in Table 2. Subgroup analyses were conducted using average age groups (≤65, >65 years), age differences between groups, current and former exposure to smoking, and the quality of the studies. Both average age groups had higher death rate than never smoker. For the disease severity among current smokers, the OR for the random-effects model in the different age groups was 1.97 (95% CI: 1.21–3.22, p=0.007) and 1.41 (95% CI: 1.01–1.97, p=0.046) in similar age groups. For the disease severity among former smokers, the OR for the random-effects model in the different age groups was 1.77 ( 95% CI: 1.22–2.58, p=0.003) and 3.05 (95% CI: 1.11–8.37, p=0.030) in similar age groups. For death among current smokers, the OR for the random-effects model in the different age groups was 1.53 (95% CI: 1.23–1.90, p<0.001). For the death among former smokers, the OR for the randomeffects model in the different age groups was 2.54 (95% CI: 2.10–3.08, p<0.001). While the death OR from the random-effects model in the stars ≥7 group (NOS quality of study) was 1.86 (95% CI: 1.35–2.55, p<0.001) and 1.52 (95% CI: 1.14–2.02, p=0.004) for stars <7 group. The severity OR from the randomeffects model in the stars <7 group was 2.17 (95% CI: 1.57–3.00, p<0.001) (Table 2).
Figure 2
Forest plots showing odds ratio of disease severity (A) and death (B) among younger smokers (≤65 years)
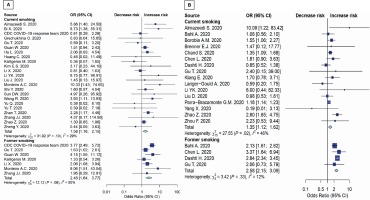
Table 1
General characteristics of 40 studies included
| Author and Year | Location | Study design | Baseline participant characteristics | Type of smoker a | Outcomes measures | OR (95% CI) | Quality of studies b | |
|---|---|---|---|---|---|---|---|---|
| Participants | Age (years) Median or Mean (SD) | |||||||
| Almazeedi S. (2020) | Kuwait | Retrospective cohort study | 1096 | 41 | Current | Disease severity Death | 5.86 (1.40–24.47) 10.09 (1.22–83.40) | 7/9 |
| Bahl A. (2020) | USA | Multicenter cohort study | 1461 | 62 | Current Former | Death | 1.08 (0.54–2.04) 2.13 (1.61–2.82) | 6/9 |
| Bellan M. (2020) | Italy | Retrospective study | 312 | 71 | Current | Death | 2.28 (1.18–4.35) | 7/9 |
| Bi X. (2020) | China | Retrospective study | 113 | 46 | Current | Disease severity | 8.73 (1.49–59.80) | 6/9 |
| Borobia A. M. (2020) | Spain | Retrospective study | 2226 | 61 | Current | Death | 1.55 (1.05–2.25) | 6/9 |
| Brenner E.J. (2020) | USA and other countries | Retrospective study | 525 | 41 | Current | Death | 1.47 (0.12–17.53) | 6/9 |
| Castelnuovo A.D. (2020) | Italy | Retrospective observational study | 1842 | 67 (12.96) | Current | Death | 1.09 (0.47–2.49) | 7/9 |
| CDC response team (2020) | USA | Retrospective study | 6637 | ≥19 | Current Former | Disease severity | 0.81 (0.26–1.99) 3.77 (2.46–5.65) | 5/9 |
| Chand S. (2020) | USA | Retrospective study | 300 | 58.2 (12.6) | Current | Death | 1.35 (1.09–1.68) | 6/9 |
| Chen L. (2020) | China | Retrospective study | 1859 | 59 | Current Former | Death | 1.81 (0.87–3.50) 3.37 (1.59–6.74) | 8/9 |
| Dashti H. (2020) | USA | Retrospective study | 12347 | 48 | Current Former | Death | 0.85 (0.51–1.34) 2.84 (2.34–3.46) | 6/9 |
| Grechukhina O. (2020) | USA | Retrospective cohort study | 141 | 30 | Current | Disease severity | 0.83 (0.02–7.11) | 7/9 |
| Gu T. (2020) | USA | Retrospective cohort study | 766 | 47 | Current Current Former Former | Disease severity Death Disease severity Death | 0.59 (0.11–3.23) 2.40 (0.15–39.60) 1.63 (1.02–2.61) 2.06 (0.73–5.77) | 8/9 |
| Guan W. (2020) | China | Retrospective study | 1085 | 47 | Current Former | Disease severity | 1.51 (0.93–2.40) 4.15 (1.51–10.90) | 6/9 |
| Hu L. (2020) | China | Retrospective study | 323 | 61 | Current | Disease severity | 2.06 (0.96–4.66) | 6/9 |
| Huang C. (2020) | China | Retrospective study | 41 | 49 | Current | Disease severity | 0.46 (0.01–5.40) | 6/9 |
| Kalligeros M. (2020) | USA | Retrospective study | 103 | 60 | Current Former | Disease severity | 0.36 (0.06–1.59) 1.33 (0.54–3.24) | 8/9 |
| Kim E.S. (2020) | Korea | Retrospective study | 28 | 42.6 (13.4) | Current | Disease severity | 3.17 (0.19–37.39) | 5/9 |
| Kishaba T. (2020) | Japan | Single-center retrospective cohort study | 7 | 74 | Current | Death | 0.13 (0.00–3.08) | 6/9 |
| Klang E. (2020) | USA | Retrospective study | 572 | 46.5 | Current | Death | 1.70 (0.80–3.80) | 8/9 |
| Langer-Gould A. (2020) | USA | Retrospective cohort study | 93 | 59.3 | Current | Death | 0.59 (0.20–1.68) | 7/9 |
| Li X. (2020) | China | Ambispective cohort study | 548 | 60 | Current Former | Disease severity | 0.81 (0.4–1.61) 2.06 (1.09–3.99) | 6/9 |
| Li YK. (2020) | China | Retrospective study | 25 | 51 | Current | Disease severity Death | 8.75 (0.89–113.30) 6.00 (0.47–87.66) | 6/9 |
| Liu D. (2020) | China | Retrospective study | 599 | 63 | Current | Death | 0.98 (0.52–1.78) | 6/9 |
| Liu J. (2020) | China | Retrospective study | 40 | 48.7 | Current | Disease severity | 1.45 (0.12–14.56) | 6/9 |
| Monteiro A.C. (2020) | USA | Retrospective observational cohort study | 112 | 61 | Current Former | Disease severity | 10.33 (1.43–74.67 8.06 (1.51–43.06) | 6/9 |
| Parra-Bracamonte G. M. (2020) | Mexico | Retrospective study | 331298 | 44 | Current | Death | 1.18 (1.13–1.22) | 6/9 |
| Shi Y. (2020) | China | Retrospective study | 487 | 46 | Current | Disease severity | 1.60 (0.52–4.17) | 6/9 |
| Sun DW. (2020) | China | Retrospective study | 57 | 64 | Current | Disease severity | 4.97 (0.61–227.20) | 6/9 |
| Torres-Macho J. (2020) | Spain | Retrospective observational study | 1968 | 67 | Current | Death | 2.44 (1.89–3.17) | 6/9 |
| Wang R. (2020) | China | Retrospective study | 125 | 42 | Current | Disease severity | 3.93 (1.08–13.56) | 6/9 |
| Yang X. (2020) | China | Retrospective observational study | 52 | 51.9 | Current | Death | 0.19 (0.01–2.66) | 6/9 |
| Yu Q. (2020) | China | Multicenter cohort study | 421 | 48 | Current | Disease severity | 0.38 (0.01–2.58) | 7/9 |
| Yu T. (2020) | China | Cross-sectional multicenter clinical study | 95 | 40 (15.88) | Current | Disease severity | 0.39 (0.01–3.40) | 6/9 |
| Zhan T. (2020) | China | Retrospective study | 405 | 56 | Current | Disease severity | 2.28 (1.17–4.47) | 6/9 |
| Zhang JJ. (2020) | China | Retrospective study | 140 | 57 | Current Former | Disease severity | 4.37 (0.34–232.00) 1.95 (0.31–13.78) | 6/9 |
| Zhao Z. (2020) | USA | Retrospective study | 641 | 60 | Current | Death Disease severity | 2.80 (1.64–4.72) 1.30 (0.85–1.97) | 7/9 |
| Zheng Y. (2020) | China | Retrospective study | 73 | 43 | Current | Disease severity | 0.44 (0.04–2.73) | 6/9 |
| Zhou F. (2020) | China | Retrospective cohort study | 191 | 56 | Current | Death | 2.23 (0.51–9.17) | 6/9 |
| Zinellu A. (2020) | Italy | Retrospective study | 94 | 72 | Current Former | Death | 0.88 (0.29–2.55) 0.99 (0.15–4.80) | 7/9 |
Table 2
Sensitivity and subgroup analyses
Meta-regression was performed to investigate the following potential moderator variables: age (>65 years), hypertension and diabetes mellitus. No significant moderators of primary and secondary outcomes with studies contributing data emerged, including age >65 years, hypertension, and diabetes mellitus (Supplementary file Table S5).
Publication bias of included studies
An appraisal of publication bias was conducted. There was no apparent publication bias as determined by the symmetric funnel plot, and Begg’s and Egger’s tests revealed no significant difference in all age groups and all outcomes (Supplementary file Figures S1–S6).
DISCUSSION
Summary of evidence
Both current and former smoking significantly increase the risk of disease severity (OR=1.58; 95% CI: 1.16–2.15, p=0.004; and OR=2.48; 95% CI: 1.64–3.77, p<0.001; respectively). Moreover, both current and former smoking also significantly increase the mortality risk among ≤65 years COVID-19 patients (OR=1.35; 95% CI: 1.12–1.62, p=0.002; and OR=2.58; 95% CI: 2.15–3.09, p<0.001; respectively).
We performed a comprehensive SR/MA to assess the possible association between disease severity and death among smokers with COVID-19. According to our analysis, with the biggest sample size, smoking is a risk factor for disease severity and death in COVID-19 patients. Current smokers have 1.58 times the odds of disease severity than never smokers. Remarkably, former smokers have 2.48 times odds of disease severity than never smokers. For death outcome, current and former smoking also significantly increase the risk of death by 1.35 and 2.58 times, respectively.
The most likely mechanism for the potential increase in the risk might be associated with the angiotensin II conversion enzyme-2 (ACE2) receptor, which is in the mucosal epithelial cell and lung alveolar tissue and found to be related to infections with COVID-19. The infection by the host virus attaching to the ACE2 receptors is probably a key step for coronavirus infection. The ACE2 gene expression is heightened in both current and former smokers compared to never smokers in a sample of patients with lung adenocarcinoma, after adjusting for age, gender, and ethnicity5,6,69. This might be a reason why former smokers have higher odds of negative outcomes than never smokers. On the contrary, the findings indicated that current smoking was less likely to have negative outcomes compared with former smoking. These might be due to the following reasons. First, the under-reporting of the current smoking status. Most studies reported smoking history instead of current smoking, which might include former smokers and therefore underestimate current smoking status among COVID-19 patients70. Second, former smokers have longer exposure period or accompanying diseases such as asthma, COPD due to smoking18. As a result, former smoking showed higher risk of negative outcomes compared with current smoking.
Although a previous systematic review examined the association between smoking and overall negative outcomes among COVID-19 patients, it was limited to only Chinese patients12. Another systematic review did not summarize the results as a meta-analysis13. One study demonstrated only the prevalence of smokers among patients hospitalized with COVID-1971 while in another study, the authors retrieved the studies from only one database and the definition of smoking was unclear8. One focused on chronic obstructive pulmonary disease (COPD) and ongoing smoking history17. One meta-analysis included just four selected studies of fair quality, which found that current smokers were more likely to develop severe COVID-19 illness compared to never smokers. But no significant difference was observed between former and never smokers. They also conducted a meta-analysis using two studies deemed to be of fair quality. So they found no significant difference between the risk of death from COVID-19 either between current and never smokers, or former and never smokers11. Finally, all literature collected did not exclude people aged >65 years, which could be a disruptive variable to the study results.
The research question requires well-designed population-based studies that control for age and relevant underlying risk factors. To our best knowledge, this study is the first comprehensive meta-analysis to assess the potential association between former and current smokers and negative outcomes of COVID-19, with the biggest sample size.
Strengths and limitations
This study has several strengths. First, we performed a comprehensive search of major databases (Embase, PubMed, Science Direct, Google Scholar and Cochrane), which is a standard method for conducting a systematic review. Second, we employed a comprehensive search strategy with no restrictions on language and study design. Third, this meta-analysis adheres to the standard methodology of systematic reviews and meta-analyses as required by the PRISMA checklist. Fourth, our study covered updated evidence and was conducted using the appropriate statistical methods for analysis. Finally, the robustness including sensitivity-analysis, subgroup-analysis and meta-regression illustrated that the results remain unchanged.
The study also has some limitations. First, all studies included were observational studies which might have residual confounders; however, this kind of study design reflects a real-world situation for evaluating the association between smoking and disease severity or death in COVID-19 patients. We also used adjusted data from the included studies as much as possible. Nevertheless, there were only non-adjusted data available in some studies. Thus, the residual confounders might distort associations and conclusions. For example, obesity, diabetes, hypertension, asthma and age were reported to increase the risk of severity of COVID-1972–74. We, therefore, analyzed using meta-regression and found that the conclusion remained the same. Second, we searched five major databases, which might not have covered all relevant studies. Nonetheless, after applying Begg’s test, Egger’s test, and a funnel plot, we found no evidence of publication bias. Third, the definitions of severity in each study were slightly different and this is a broad exploratory meta-analysis, which might distort the association between smoking and outcome in COVID-19 patients. Therefore, the results should be interpreted cautiously. However, from another perspective, the effects of smoking in our analysis were consistent across studies, which may indicate high generalizability of the results to any circumstances. Fourth, even key important factors that may potentially affect our findings were number of cigarettes smoked, nicotine addiction level, and the length of time after quitting until COVID-19 infection, which were not reported in the included studies. Nevertheless, our comprehensive sensitivity analysis showed a negative association of smoking on the outcomes.
Further research directions
Well-designed longitudinal population-based studies are needed to address questions about the risk of infection by SARS-CoV-2 and the risk of hospitalization with COVID-19. Stronger evidence coming from smoking status data that are systemically recorded and analyzed among COVID-19 patients are needed. Some factors such as number of cigarettes smoked, nicotine addiction level, and the length of time after quitting until COVID-19 infection should be collected.
CONCLUSIONS
Smoking is confirmed to be a risk factor for the negative progression of COVID-19, particularly on disease severity and death. Both current and former smokers have higher odds of disease severity than never smokers. Given the well-established harm associated with tobacco use, smoking cessation is recommended for all smokers and avoidance of secondhand smoke by non-smokers.