INTRODUCTION
Obstructive sleep apnea (OSA) is the most common sleep-related breathing disorder. The prevalence of OSA in China, Spain, Korea, Sweden, the United States, Japan, and India between 1993 and 2013 was 22% and 17% in males and females, respectively1. Over 90% of patients with OSA are misdiagnosed; therefore, most of them remain untreated2. OSA is a growing health problem that is associated with various complications such as daytime fatigue, neurocognitive effects, headaches on awakening, fetal growth retardation, and disruption of bed partners’ sleep quality. If left untreated, OSA can be accompanied by glaucoma, cardiovascular events, stroke, and pulmonary hypertension3. Therefore, to prevent health deterioration due to the complications of OSA, identifying the risk factors for OSA is an important public health challenge.
The gold standard for diagnosing OSA is by using an overnight polysomnogram; however, it is time-consuming, expensive, and requires a professional examiner. Therefore, several alternate, simple, and reliable methods were devised to identify individuals at high risk for OSA4-6. However, even these were complex or time-consuming and required upper airway evaluation. The STOP-Bang questionnaire, developed in 2008, is a simple and useful tool for screening OSA risk groups7. The STOP-Bang questionnaire is a self-reported questionnaire with four yes/no questions about snoring, tiredness, observed apnea, and high blood pressure, and four additional demographic questions concerning BMI (≥35 kg/m2), age (>50 years), neck circumference (>40 cm), and sex (male). Each question is assigned 1 point; thus, the total number of points ranges 0–8. Originally, the STOP-Bang scores were classified as: ≥3 for moderate-to-severe risk, and <3 for low risk of OSA. This tool has a sensitivity of ≥84%, 93%, and 100% and a specificity of 56.4%, 43%, and 37% for detecting any OSA, moderate to severe OSA, and severe OSA, respectively8. Owing to its high sensitivity and simplicity, STOP-Bang has been widely used in diverse populations8,9.
Male sex, older age, familial aggregation, obesity, central body fat distribution, large neck girth, upper airway abnormalities, congestive heart failure, type 2 diabetes mellitus, and stroke are well-known risk factors for sleep apnea10. Several recent studies have found an association between smoking and alcohol use and sleep apnea or snoring11. Current and former smoking, as well as heavy smoking, are associated with OSA12. The effects of smoking on OSA and their mechanisms have been studied; although it is still a hypothesis, the mechanisms may include alterations in sleep architecture, impairment of upper airway neuromuscular function, higher arousal index, and upper airway inflammation14. However, only a few studies have investigated the association between OSA and electronic cigarette (e-cigarette) use, even though e-cigarettes are becoming increasingly popular14. Since e-cigarettes are considered healthier alternatives to cigarettes, their use is increasing; hence, they are used to reduce or quit cigarette smoking16. Despite such beliefs, there is still a lack of information about smoking risks according to the type of cigarette, including electronic cigarettes. Thus, we investigated the association between smoking behavior including smoking status, types of cigarettes and OSA, as determined by the STOP-Bang score.
METHODS
Study population and data
This secondary dataset analysis was performed on the cross-sectional 2019–2021 Korean National Health and Nutrition Examination Survey (KNHANES) because the STOP-Bang questionnaire was administered only during this period. The KNHANES is a survey of national health conditions, nutritional intake, and dietary habits conducted by the Korea Disease Control and Prevention Agency (KCDA) in Korea. More information about the design and content of the KNHANES can be found on its webpage (knhanes. kdca. go. kr/knhanes/eng/index. do). The KNHANES protocols were approved by the Institutional Review Board of the KCDA (IRB No. 2018-01-03-C-A), and the research complied with the tenets of the Declaration of Helsinki for medical research involving human subjects. Informed consent was obtained from all the participants. Since the KNHANES is a survey conducted by the government for public welfare, ethics approval for the secondary dataset analysis of KNHANES is waived by the Bioethics and Safety Act of 2015.
A total of 22559 participants were included in the 2019–2021 KNHANES. As the survey on snoring, tiredness, and observed apnea was not conducted for respondents aged <40 years, only participants aged ≥40 years were included. Among them, 7350 participants (3226 male and 4124 female) were included in the present study after excluding participants with missing data.
STOP-Bang score
The STOP-Bang score was used to screen for OSA. This questionnaire comprises eight items. Each item receives 1 point when conditions are satisfied, and the types of conditions are: 1) loud snoring, 2) daytime tiredness, 3) onlooker-observed cessation of breathing during sleep, 4) high blood pressure (BP) or consumption of medication for high BP, 5) body mass index (BMI) ≥35 kg/m2, 6) age >50 years, 7) neck circumference >40 cm, and 8) male sex. In this study, the STOP-Bang algorithm with a 2-step scoring strategy was used to identify the association between the high-risk groups for OSA and smoking behavior. In the first step, those with STOP-Bang scores ≥5 were classified as the high-risk group for OSA. Subsequently, among the intermediate group with STOP-Bang scores of 3–4, participants who were male, had a BMI >35 kg/m2, or neck circumferences >40 cm were also classified as the high-risk group (second step)16.
Smoking behavior
The main independent variable in this study was smoking behavior which comprised current cigarette or e-cigarette smoking and a history of smoking. Individuals who used both e-cigarettes and cigarettes during their lifetime were classified as dual users, whereas those who used cigarettes only were classified as cigarette only smokers. E-cigarette users included users of e-cigarettes or liquid e-cigarettes containing nicotine. Because only a very small number of participants used e-cigarettes only during their lifetime (n=1) and this study was aimed at deriving the association between vaping and sleep disorders, we included the e-cigarette only users in the dual users. Finally, those who never used cigarettes or e-cigarettes during their lifetime were classified as never smokers. Based on these categories, the participants were divided into three groups: dual users of e-cigarettes and cigarettes, cigarette only users, and never smokers.
For the subgroup analysis, never smokers were classified according to exposure to secondhand smoke, and dual users and cigarette only users were classified according to the types of cigarettes used in the past and present. Specifically, current smokers were classified as those who had used cigarettes or e-cigarettes within the past month, and past smokers were classified as those who had used cigarettes or e-cigarettes throughout their lifetime but had not used them within the past month.
Covariates
The covariates in this study included cumulative smoking exposure (0, 0< and ≤30, 30< and ≤60, or >60 pack-years); exposure to passive smoking (yes, no); sex (male, female); age (40–49, 50–59, 60–69, ≥70 years); education level (lower than college, college or higher); occupation (white, pink, blue collar, none); monthly household income quartile (low, middle-low, middle-high, high); marital status (yes, no); alcohol consumption (2–4 times/week, 2–4 times/month, never or occasionally); body mass index (BMI) (<23, 23 ≤ and < 25, 25 ≤ and < 27.5, 27.5 ≤ and < 30, or ≥30 kg/m2); muscle strengthening activity (low or high, with high indicating strength exercises such as dumbbells, weights, and iron bars at least twice a week); aerobic activity (low or high, with high indicating >30 min of aerobic activity ≥5 days per week)15; and history of diabetes (no, prediabetes, diabetes), hypertension (no, pre-hypertension, hypertension), asthma (no, yes), stroke (no, yes), and myocardial infarction (no, yes). Prediabetes was defined as having a fasting blood sugar of 100–125 mg/dL or hemoglobin A1c of 5.7–6.4%17, and pre-hypertension was defined as having a systolic blood pressure of 120 ≤ and <140 mmHg and diastolic blood pressure of 80≤ and <90 mmHg18.
Statistical analysis
A multiple logistic regression analysis was conducted to examine the relationship between smoking behavior and a high risk of OSA using the STOP-Bang score after adjusting for all covariates. The results are given as adjusted odds ratio (AOR) and 95% confidence interval (CI). A subgroup analysis was also conducted to identify the association between dual cigarette use in the past and present with a high risk of OSA after adjusting for covariates. Finally, stratified analyses were performed through multiple logistic regression to examine the association between smoking behavior and high risk of OSA using the 2-stage STOP-Bang score according to sex, aerobic activity, muscle strengthening activity, and BMI.
All analyses were performed using Statistical Analysis Software version 9.4 (SAS Institute, Cary, NC, USA), and a weighted logistic regression analysis was used to treat the complex and stratified sampling design15. The KNHANES provided weights for individual-level analysis to ensure that the survey participants represent the entire population of Korea. Two-sided p<0.05 was considered statistically significant. This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline for cross-sectional studies19.
RESULTS
Table 1 presents the general characteristics of the study participants. Among the 7350 participants, 417 (5.7%) had a high risk for OSA according to the 2-stage STOP-Bang score. The number of participants who were dual users (e-cigarettes and cigarettes) was 431 (5.9%), cigarette only users were 2520 (34.3%), and never smokers were 4399 (59.9%). A high risk of OSA was observed in 49 (11.4%) of the 431 dual users and in 290 (11.5%) of the 2520 cigarette only users. Among the 4399 never smokers, 78 (1.8%) had a high risk of OSA, according to the STOP-Bang score.
Table 1
General characteristics of study subjects, 2019–2021 Korean National Health and Nutrition Examination Survey
| Characteristics | STOP-Bang score for OSA | p* | |||||
|---|---|---|---|---|---|---|---|
| Total | Low/intermediate risk | High risk | |||||
| n | % | n | % | n | % | ||
| Total | 7350 | 100 | 6933 | 94.3 | 417 | 5.7 | |
| Smoking behavior | <0.001 | ||||||
| Dual user (e-cig + cig) | 431 | 5.9 | 382 | 88.6 | 49 | 11.4 | |
| Cigarette only user | 2520 | 34.3 | 2230 | 88.5 | 290 | 11.5 | |
| Never smoker | 4399 | 59.9 | 4321 | 98.2 | 78 | 1.8 | |
| Cumulative smoking exposure (pack-years) | <0.001 | ||||||
| 0 | 4517 | 61.5 | 4427 | 98.0 | 90 | 2.0 | |
| 0< and ≤30 | 2169 | 29.5 | 1935 | 89.2 | 234 | 10.8 | |
| 30< and ≤60 | 574 | 7.8 | 495 | 86.2 | 79 | 13.8 | |
| >60 | 90 | 1.2 | 76 | 84.4 | 14 | 15.6 | |
| Exposure to secondhand smoke | <0.001 | ||||||
| No | 6355 | 86.5 | 6030 | 94.9 | 325 | 5.1 | |
| Yes | 995 | 13.5 | 903 | 90.8 | 92 | 9.2 | |
| Sex | <0.001 | ||||||
| Male | 3226 | 43.9 | 2845 | 88.2 | 381 | 11.8 | |
| Female | 4124 | 56.1 | 4088 | 99.1 | 36 | 0.9 | |
| Age (years) | 0.001 | ||||||
| 40–49 | 1748 | 23.8 | 1658 | 94.9 | 90 | 5.1 | |
| 50–59 | 1884 | 25.6 | 1754 | 93.1 | 130 | 6.9 | |
| 60–69 | 1980 | 26.9 | 1854 | 93.6 | 126 | 6.4 | |
| ≥70 | 1738 | 23.6 | 1667 | 95.9 | 71 | 4.1 | |
| Education level | 0.095 | ||||||
| Lower than college | 5013 | 68.2 | 4744 | 94.6 | 269 | 5.4 | |
| College or higher | 2337 | 31.8 | 2189 | 93.7 | 148 | 6.3 | |
| Region | 0.075 | ||||||
| Rural | 4133 | 56.2 | 3881 | 93.9 | 252 | 6.1 | |
| Metropolitan | 3217 | 43.8 | 3052 | 94.9 | 165 | 5.1 | |
| Employment | <0.001 | ||||||
| White collar | 1474 | 20.1 | 1353 | 91.8 | 121 | 8.2 | |
| Pink collar | 921 | 12.5 | 882 | 95.8 | 39 | 4.2 | |
| Blue collar | 1987 | 27.0 | 1843 | 92.8 | 144 | 7.2 | |
| None | 2968 | 40.4 | 2855 | 96.2 | 113 | 3.8 | |
| Household income | 0.051 | ||||||
| Low | 1524 | 20.7 | 1455 | 95.5 | 69 | 4.5 | |
| Middle low | 1819 | 24.7 | 1721 | 94.6 | 98 | 5.4 | |
| Middle high | 1961 | 26.7 | 1830 | 93.3 | 131 | 6.7 | |
| High | 2046 | 27.8 | 1927 | 94.2 | 119 | 5.8 | |
| Marital status | 0.001 | ||||||
| Not married | 1699 | 23.1 | 1632 | 96.1 | 67 | 3.9 | |
| Married | 5651 | 76.9 | 5301 | 93.8 | 350 | 6.2 | |
| Alcohol consumption | <0.001 | ||||||
| 2–4 times/week | 1430 | 19.5 | 1284 | 89.8 | 146 | 10.2 | |
| 2–4 times/month | 1341 | 18.2 | 1249 | 93.1 | 92 | 6.9 | |
| Never or occasionally | 4579 | 62.3 | 4400 | 96.1 | 179 | 3.9 | |
| BMI (kg/m2) | <0.001 | ||||||
| <23 | 2712 | 36.9 | 2681 | 98.9 | 31 | 1.1 | |
| 23≤ and <25 | 1801 | 24.5 | 1746 | 96.9 | 55 | 3.1 | |
| 25≤ and <27.5 | 1670 | 22.7 | 1558 | 93.3 | 112 | 6.7 | |
| 27.5≤ and <30 | 744 | 10.1 | 631 | 84.8 | 113 | 15.2 | |
| ≥30 | 423 | 5.8 | 317 | 74.9 | 106 | 25.1 | |
| Aerobic activity | 0.136 | ||||||
| Low | 5984 | 81.4 | 5633 | 94.1 | 351 | 5.9 | |
| High | 1366 | 18.6 | 1300 | 95.2 | 66 | 4.8 | |
| Muscle strengthening activity | 0.677 | ||||||
| Low | 5788 | 78.7 | 5463 | 94.4 | 325 | 5.6 | |
| High | 1562 | 21.3 | 1470 | 94.1 | 92 | 5.9 | |
| Diabetes | <0.001 | ||||||
| No | 2210 | 30.1 | 2153 | 97.4 | 57 | 2.6 | |
| Prediabetesa | 3613 | 49.2 | 3414 | 94.5 | 199 | 5.5 | |
| Diabetes | 1527 | 20.8 | 1366 | 89.5 | 161 | 10.5 | |
| Hypertension | <0.001 | ||||||
| No | 2527 | 34.4 | 2493 | 98.7 | 34 | 1.3 | |
| Pre-hypertensionb | 1788 | 24.3 | 1726 | 96.5 | 62 | 3.5 | |
| Hypertension | 3035 | 41.3 | 2714 | 89.4 | 321 | 10.6 | |
| Asthma | 0.510 | ||||||
| No | 7109 | 96.7 | 6708 | 94.4 | 401 | 5.6 | |
| Yes | 241 | 3.3 | 225 | 93.4 | 16 | 6.6 | |
| Stroke | 0.411 | ||||||
| No | 7150 | 97.3 | 6747 | 94.4 | 403 | 5.6 | |
| Yes | 200 | 2.7 | 186 | 93.0 | 14 | 7.0 | |
| Myocardial infarction | 0.001 | ||||||
| No | 7235 | 98.4 | 6833 | 94.4 | 402 | 5.6 | |
| Yes | 115 | 1.6 | 100 | 87.0 | 15 | 13.0 | |
Table 2 shows the factors associated with a high risk of OSA through multiple logistic regression analysis. Compared to those who were never smokers, cigarette only users and dual users had higher risks of increased OSA (cigarette only users, AOR=2.75; 95% CI: 1.33–5.68 and dual users, AOR=2.45; 95% CI: 1.04–5.79). Other factors that increased the probability of high risk for OSA were male sex, obesity, a history of diabetes, and hypertension.
Table 2
Factors associated with high risk of obstructive sleep apnea assessed by STOP-Bang score, 2019–2021 Korean National Health and Nutrition Examination Survey
| Variables | High risk of OSA | ||
|---|---|---|---|
| AOR | 95% CI | p* | |
| Smoking behavior | |||
| Dual user (e-cig + cig ) | 2.45 | 1.04–5.79 | 0.042 |
| Cigarette only user | 2.75 | 1.33–5.68 | 0.006 |
| Never smoker (Ref.) | 1.00 | ||
| Cumulative smoking exposure (pack-years) | |||
| 0 (Ref.) | 1.00 | ||
| 0< and ≤30 | 0.74 | 0.37–1.51 | 0.407 |
| 30< and ≤60 | 0.67 | 0.31–1.44 | 0.306 |
| >60 | 1.20 | 0.44–3.24 | 0.722 |
| Exposure to secondhand smoke | |||
| No | 1.00 | ||
| Yes | 1.30 | 0.92–1.83 | 0.141 |
| Sex | |||
| Male | 9.40 | 5.61–15.75 | <0.001 |
| Female (Ref.) | 1.00 | ||
| Age (years) | |||
| 40–49 (Ref.) | 1.00 | ||
| 50–59 | 1.93 | 1.32–2.81 | 0.001 |
| 60–69 | 1.50 | 0.97–2.32 | 0.066 |
| ≥70 | 1.03 | 0.62–1.70 | 0.980 |
| Education level | |||
| Lower than college | 1.16 | 0.84–1.59 | 0.362 |
| College or higher (Ref.) | 1.00 | ||
| Region | |||
| Rural | 1.16 | 0.88–1.54 | 0.298 |
| Metropolitan (Ref.) | 1.00 | ||
| Employment | |||
| White collar | 1.67 | 1.08–2.59 | 0.023 |
| Pink collar | 1.01 | 0.60–1.69 | 0.978 |
| Blue collar | 0.87 | 0.58–1.30 | 0.505 |
| None (Ref.) | 1.00 | ||
| Household income | |||
| Low | 1.11 | 0.67–1.81 | 0.692 |
| Middle low | 1.09 | 0.74–1.61 | 0.658 |
| Middle high | 1.32 | 0.92–1.89 | 0.130 |
| High (Ref.) | 1.00 | ||
| Marital status | |||
| Not married | 0.80 | 0.55–1.15 | 0.220 |
| Married (Ref.) | 1.00 | ||
| Alcohol consumption | |||
| 2–4 times/week | 1.08 | 0.74–1.60 | 0.683 |
| 2–4 times/month (Ref.) | 1.00 | ||
| Never/occasionally | 1.31 | 0.90–1.91 | 0.159 |
| BMI (kg/m2) | |||
| <23 (Ref.) | 1.00 | ||
| 23 ≤ and <25 | 1.15 | 0.66–2.01 | 0.624 |
| 25≤ and <27.5 | 3.07 | 1.88–5.01 | <0.001 |
| 27.5≤ and <30 | 6.66 | 4.10–10.82 | <0.001 |
| ≥30 | 17.10 | 9.83–29.75 | <0.001 |
| Aerobic activity | |||
| Low | 1.11 | 0.77–1.59 | 0.590 |
| High (Ref.) | 1.00 | ||
| Muscle strengthening activity | |||
| Low | 1.04 | 0.73–1.47 | 0.842 |
| High (Ref.) | 1.00 | ||
| Diabetes | |||
| No (Ref.) | 1.00 | ||
| Prediabetesa | 0.93 | 0.64–1.36 | 0.715 |
| Diabetes | 1.73 | 1.16–2.58 | 0.007 |
| Hypertension | |||
| No (Ref.) | 1.00 | ||
| Pre-hypertensionb | 1.58 | 0.94–2.67 | 0.086 |
| Hypertension | 5.57 | 3.61–8.59 | <0.001 |
| Asthma | |||
| No (Ref.) | 1.00 | ||
| Yes | 0.98 | 0.50–1.92 | 0.963 |
| Stroke | |||
| No (Ref.) | 1.00 | ||
| Yes | 0.77 | 0.37–1.62 | 0.494 |
| Myocardial infarction | |||
| No (Ref.) | 1.00 | ||
| Yes | 1.13 | 0.59–2.16 | 0.706 |
Figure 1 shows the results of the subgroup analysis. Individuals who did not currently smoke but had a history of dual use were associated with a significant risk of OSA (AOR=3.61; 95% CI: 1.32–9.92). Furthermore, former cigarette smoking was associated with the likelihood of OSA (AOR=3.66; 95% CI: 1.63–8.23). Individuals who were dual users in the past and the present had increased odds of OSA, although this was not statistically significant.
Figure 1
Subgroup analysis of the association of previous and current cigarette type with the risk of obstructive sleep apnea using STOP-Bang score
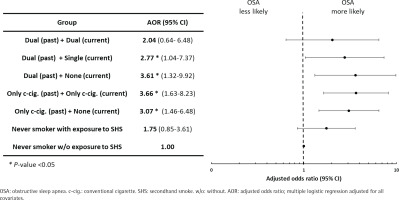
Table 3 presents the results of the stratified analyses according to the independent variables. The probability of a high risk of OSA was 7.35 times higher for females with dual use (AOR=7.35; 95% CI: 1.04–59.80), but this was not significant for males with dual use. The association between the dual use of e-cigarettes and cigarettes and OSA was prominent in the those with low aerobic activity (AOR=2.04; 95% CI: 1.11–3.76) and low muscle strengthening activity (AOR=2.27; 95% CI: 1.17–4.37), and those with high-risk obesity (BMI >30 kg/m2) (AOR=4.98; 95% CI: 1.47–16.86). We observed a significant multiplicative interaction between smoking behavior and BMI (p<0.001).
Table 3
Stratified analyses of a high risk of OSA versus smoking behavior, according to independent variables, 2019–2021 Korean National Health and Nutrition Examination Survey
| Variables | High risk of OSA using STOP-Bang score | ||||||
|---|---|---|---|---|---|---|---|
| Smoking behavior | |||||||
| Never smoker (Ref.) | Cigarette only user | p* | Dual user (e-cig + cig) | p* | |||
| AOR | AOR | 95% CI | AOR | 95 % CI | |||
| Sex | |||||||
| Male | 1.00 | 1.97 | 1.31–2.97 | 0.001 | 1.67 | 0.96–2.90 | 0.070 |
| Female | 1.00 | 2.15 | 0.70–6.58 | 0.178 | 7.35 | 1.04–59.80 | 0.048 |
| Aerobic activity | |||||||
| Low | 1.00 | 2.07 | 1.34–3.20 | 0.001 | 2.04 | 1.11–3.76 | 0.023 |
| High | 1.00 | 2.86 | 1.00–8.20 | 0.048 | 0.66 | 0.14–3.14 | 0.598 |
| Muscle strengthening activity | |||||||
| Low | 1.00 | 2.34 | 1.49–3.68 | 0.001 | 2.27 | 1.17–4.37 | 0.015 |
| High | 1.00 | 1.20 | 0.56–2.58 | 0.647 | 0.75 | 0.24–2.38 | 0.621 |
| BMI (kg/m2) | |||||||
| <25 | 1.00 | 1.75 | 0.82–3.72 | 0.145 | 0.86 | 0.27–2.72 | 0.801 |
| 25≤ and <30 | 1.00 | 1.90 | 1.14–3.16 | 0.014 | 1.55 | 0.73–3.28 | 0.255 |
| ≥30 | 1.00 | 2.90 | 1.33–6.31 | 0.008 | 4.98 | 1.47–16.86 | 0.009 |
DISCUSSION
We investigated the association between the STOP-Bang score, which reflects the risk of OSA, and smoking behavior, including smoking status and types of cigarettes (e-cigarettes or cigarettes). The odds of having a high risk for OSA in the dual users and the cigarette only users were significantly higher than in the never smokers. Interestingly, we observed a significantly increased risk for OSA in participants who did not currently smoke but had previously were dual users or cigarette only users.
Several hypotheses may explain our result concerning cigarettes, although the mechanisms are still unclear13. First, alterations in sleep architecture caused by smoking may increase the risk of OSA. This hypothesis was based on a prospective study using electroencephalogram spectrum analysis, which found that smokers’ sleep quality was inferior to that of non-smokers, especially in the early stages of sleep20. Another study using polysomnography showed that smokers had a higher rapid eye movement sleep density, shorter sleep time, and longer sleep latency21. Second, the impairment of upper airway neuromuscular function in smokers can lead to a higher risk of OSA. However, the relationship between nicotine use and upper airway damage has only been demonstrated in animal experiments22. In humans, it is unclear whether smoking causes upper airway damage, although it has been shown that smoking can reduce reflexive mechanisms after damage23. Third, arousal mechanisms may increase the risk of developing OSA. Smokers have a higher arousal index, which can result in increased sleep instability24. Lastly, upper airway inflammation can lead to a higher risk of OSA. Smoking can cause airway inflammation and, consequently, upper airway narrowing25.
Although the association between smoking and OSA has been investigated, only a few studies have addressed the association between dual use of e-cigarettes and cigarettes and OSA. However, as more people are using e-cigarettes to quit smoking, and OSA is a growing health problem, awareness of the need for studies on the relationship between e-cigarettes and OSA is gradually increasing. Therefore, in this study, in addition to the association between cigarette smoking and a higher likelihood of OSA, we investigated the association between dual use of cigarettes and OSA. E-cigarettes cause various health problems because e-cigarette aerosols contain highly oxidized freebase nicotine26. Additionally, flavoring materials in e-cigarettes are associated with cytotoxicity27, and toxic compounds such as carbonyls, metals, volatile organic compounds, and nitrosamines are found in almost all e-cigarettes28,29. Nicotine withdrawal can also lead to sleep instability, which can predispose patients to upper airway obstruction30. However, considering that our study investigating the association between dual use and the risk of OSA is a cross-sectional study and that the dual users may include many e-cigarette experimenters, the above interpretation requires caution. Cohort studies that can ensure causality are needed.
Despite the knowledge of the effects of e-cigarettes, to the best of our knowledge, Brett et al.31 in 2020 were the first to investigate the association between e-cigarettes and sleep disturbance; therefore, the relationship between e-cigarettes and sleep disorders and the mechanism by which e-cigarette use causes sleep disorders is still unclear. According to a previous study, even the sporadic use of e-cigarettes can cause sleep disturbances due to high nicotine exposure. On average, more e-cigarette smokers take sleeping medications than cigarette smokers31. Although these hypotheses support the association of dual use with the high risk of OSA, participants who smoked both e-cigarettes and cigarettes, both in the past and present, tended to have a higher risk of OSA than those who never smoked; however, this was not significant. These results could be due to the small number of respondents in these groups, which may show a tendency but is considered insufficient to draw a significant result. Moreover, dual users of cigarettes and e-cigarettes in the past to the present may not have consumed excessive nicotine due to the control on the amount of smoking32. Further research needs to be conducted using data from more participants and e-cigarette users.
In the stratified analyses, the association between dual use and the risk of OSA was much more pronounced in females. Although the risk of OSA is lower in females than in males, there is a need to raise awareness as our study shows that dual use was associated with a higher reported likelihood of OSA in females1. Furthermore, the association between dual use and OSA was significant in those with low physical activity. Considering that there was no association between aerobic activity and muscle-strengthening activity levels and OSA, the lack of aerobic or muscle-strengthening activity and the dual use of e-cigarettes and cigarettes may exert a synergistic effect on the development of OSA. This suggests that physical activity can offset the negative effects of smoking on OSA33. In participants with a BMI > 30 kg/m2, the standard of obesity presented by the World Health Organization, mixing the types of tobacco increased the odds of having OSA by approximately five times. The strength of this association was much higher than in those with low BMI, and much higher than in cigarette only users. These results suggest that substances such as cytokines or inflammatory markers released from dual use of cigarettes can affect airway inflammation in obese individuals34.
Limitations
This study has some limitations. First, because a cross-sectional design was used, it was not possible to confirm a causal relationship. Second, the number of e-cigarette users was too small to obtain high statistical power. Third, the number of current dual users was small, making analysis difficult. Fourth, although the cumulative use of cigarettes was included in the analysis, the cumulative use of e-cigarettes was not included because of data limitations. Since e-cigarette experimenters are likely to account for the majority of e-cigarette users, cumulative use of e-cigarettes needs to be calibrated. Fifth, we investigated the association between dual use and the high-risk group for OSA through the STOP-Bang score, not using the diagnosis of OSA directly. Finally, the cutoff for dividing the risk of the STOP-Bang score differs between studies. However, we selected a high-risk group for OSA in 2 steps, and the method was found to have high validity17.
CONCLUSIONS
There are insufficient studies on the association between the types of cigarettes used and OSA, and the association between the two has not been fully elucidated. In the current study, we found that the dual use of e-cigarettes and cigarettes was significantly associated with OSA when compared to non-smoking. In addition, the dual use of cigarettes in the past was associated with a significant likelihood of OSA, even if the participants did not currently smoke or currently smoked only one product type. The association between dual use and OSA was more pronounced in females, participants with obesity, and those with a low level of physical activity. Further research is needed to confirm the causal relationship between the dual use of cigarettes and the risk of OSA.


