INTRODUCTION
Globally, prostate cancer (PCa) is the second leading cause of cancer and the fifth leading cause of cancer mortality among men worldwide1. The burden of PCa is predicted to grow by nearly around 2.3 million incidences and 740000 deaths worldwide by 20402. In the USA, there were an estimated 248530 new cases and 34130 deaths from PCa in the year 20213. The reported incidence of PCa in developing countries is lower than in the developed countries. It is not clear what the reason is; however, it might be due to underreporting from diagnosing centers to the national cancer registry and the geographical variation reflected by ethnic and racial dissimilarity4,5. Worldwide the incidence of PCa has increased remarkably, which might be attributed to the increased screening uptake among men for prostate-specific antigen (PSA) without disease symptoms6.
Few risk factors have been identified and linked with PCa, such as a well-connected factor of racial and ethnic variation affecting the incidence of PCa. African American and Hispanic males were more likely to be diagnosed with advanced-stage PCa compared with non-Hispanic White males. Similarly, recent research demonstrated that family history and genetic factors play a role in the risk of having PCa7.
Concerning age, like other types of cancer, it seems that the risk of PCa rises markedly after the age of 50 years; this might be related to the accumulated exposure to risk factors over the years. Other risk factors associated with prostate cancer but with lower evidence for a causal association, are sexually transmitted disease, unhealthy diet, inflammation, obesity, and smoking3,8.
Regarding smoking, burning tobacco and inhaling the smoke is considered a significant risk factor or direct cause of cancer, tumor lesions, and a well-known chemical carcinogen. Yet, it is still debatable whether cigarette smoking is a causative factor of PCa9,10.
Many studies have reported that smoking and prostate cancer together can lead to a higher mortality rate, especially in heavy smokers11,12. A meta-analysis on PCa death rates among smokers showed that smoking is a modifiable environmental risk factor leading to high death rates among PCa patients. However, smoking by itself is a low-risk factor for developing prostate cancer13. Similarly, De Nunzio et al.14, reported in their systematic review that smokers have a higher mortality rate and worse outcomes after treatment.
On the other hand, Huncharek et al.10 conducted a meta-analysis on 24 observational studies and found that the effect of smoking by itself on PCa incidence is weak. In fact, several cohort studies showed that smoking has a significant inverse association with PCa incidence15-19.
In contrast, Ho et al.20, in their primary research published in 2014, studied 6240 men and reported that smoking was a risk factor for developing high-grade prostate cancer20. Whereas Butler et al.21 conducted a study on 27293 Chinese and Singaporean men in 2006 and found that smoking is not considered a risk factor for developing PCa. However, the same study found a slight association between those who started smoking at an early age against those who started late.
Current epidemiological evidence shows conflicting evidence about the association between cigarette smoking and risk of PCa. To the best of our knowledge, a previous systematic review and meta-analysis conducted in 2010 showed no association between smoking and PCa10, while the latest in 2014 showed an inverse association between cigarette smoking and PCa13. Therefore, this systematic review and meta-analysis aimed to assess the association between cigarette smoking and PCa risk, based on updated cohort studies.
METHODS
Literature search strategy
The search of study of interest was done using MEDLINE and SCOPUS databases using keywords: smoking, tobacco, prostate, cancer, cigarettes, and tumor. Boolean operators (OR and AND) were used to reach published primary research that explicitly investigated this research question. The truncation * and quotation marks were used whenever appropriate to avoid missing any relevant articles when using our main keywords. In addition to keywords search, Mesh terms were exploded in PubMed to reach all articles relevant to our research question. Bibliographies were screened to conduct cross-referencing following the identification of relevant articles. Our search was limited to articles published in the English language between 2000 and October 2021. All through the search process, we followed PRISMA statement guidelines (preferred reporting item for systematic reviews and meta-analysis).
Eligibility criteria
Given that it is a systematic review and meta-analysis, our study sample was relevant published articles. Cohort study designs in the human population were included in our search. Studies that assess the risk of prostate cancer in current smokers compared to non-smokers were eligible in the meta-analysis. The minimum requirement for any study included in the meta-analysis was to that the analysis adjusted for age as a confounder. We excluded published narrative or systematic reviews, meta-analysis, case reports, letters to the editor, editorials, case-control, cross-sectional, and guidelines. The inclusion criteria of articles concerning the exposure were broad, and we accepted all types of assessments of cigarette smoking. However, the inclusion criteria for the outcome were limited to studies that used biopsy as a diagnostic tool, identification through cancer registries, and formal physician diagnosis of prostate cancer. Studies that used subjective assessment methods of occurrence of prostate cancer (e.g. by self-report directly from patients or family) were excluded.
Data extraction and quality assessment
We did not attempt to contact the authors of any of the studies to request data or any additional information. The following information was extracted from each study: the first author’s surname, publication year, location (country or region) of the conducted study, follow-up time, the total number of participants and cancer cases identified among them, age of the participants, smoking categorization used, relative risk (RR) or hazard ratio (HR) estimate for each smoking category, and the corresponding 95% confidence interval, and finally the confounder variable that was adjusted for in the analysis. Data were independently extracted by two reviewers and the quality of the included studies was assessed independently as well using the New Castle Ottawa quality assessment scale (NOS) for cohort studies. A NOS score between 0 and 9 was allocated to each study. Studies with a score of ≥7 were considered high-quality studies, while studies that scored 4–6 were considered of moderate quality, and score ≤3 of poor quality. Conflicting assessments were resolved through discussion.
Statistical analysis
The association between cigarette smoking and prostate cancer incidence and mortality was determined based on the relative risk (RR) and the 95% confidence interval (CIs) for each study. The hazard ratio (HR) is considered equivalent to (RR) in prospective cohort studies; therefore, all risk estimates were presented as relative risk.
We calculated the pooled relative risk estimate for current smokers and the risk of prostate cancer incidence and mortality. We used a random effect model when heterogeneity was significantly high and a fixed-effect model when heterogeneity diminished. The size of heterogeneity was determined by the I2 statistic, Cochran’s chi-squared (Q-test), and p<0.10 indicating significant heterogeneity. The heterogeneity points out any variability in the association between smoking and prostate cancer among diverse studies. The presence of significant heterogeneity suggests that the variation between studies is not due to chance alone. The I2 statistic ranges from 0% to 100%. The interpretation of these values falls into two categories below and above 50%, which indicate low and high heterogeneity, respectively. A sensitivity analysis was performed by excluding studies with lower quality scores and decreased follow-up time to examine the influence of such articles on the result of the crude meta-analysis. We used funnel plots for evaluating the existence of publication bias. The funnel is a scatter plot of the number of studies included in the meta-analysis; with the value of smoking effect on the horizontal axis, and the weight of the study, such as the sample size and inverse standard error, on the vertical axis. As the sample size in the studies increases, the precision in evaluating the association between smoking and prostate cancer increases. An asymmetrical pattern in the funnel plot may indicate the presence of publication bias. Statistical analyses were conducted using RevMan software (version 5.4).
RESULTS
Characteristics of studies
A Preferred Reporting Item for Systematic Reviews and Meta-Analysis (PRISMA) flow diagram that summarizes the search strategy is shown in Figure 1. The systematic literature search yielded a total of 2176 articles. After removing those with irrelevant title and or abstract, there were 21 relevant studies that assessed the association between cigarette smoking and the risk of prostate cancer incidence. We included 17 articles that met our inclusion and exclusion criteria in the systematic review schedule. The selected studies were published between 2000 and 2019. Finally, 15 articles were suitable for inclusion in the meta-analysis. From all cohorts included in this systematic review, five studies examined the association between current smokers and the risk of death from prostate cancer. Therefore, a fixed effect meta-analysis of these cohort studies was performed due to insignificant heterogeneity. Mostly, all cohorts were drawn from the general population except for two studies that represented selected groups of physicians and health professionals22,23.
Figure 1
PRISMA flowchart identifying the studies that were included in the systematic review and meta-analysis
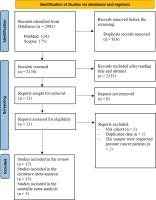
A total of 2537664 participants and 47536 incident cases were included. Seven reports (42%) were from North America11,17,18,22-25. Approximately the same proportion of studies was carried out in the United Kingdom16,26,27 and European countries19,28,29, while the remaining four studies were conducted in Asian countries15,21,30,31.
All included studies in the systematic review are summarized in Table 1. Smoking status was assessed using a questionnaire in all included studies, except one study that used an in-person interview to assess smoking status21. Identification of smoking categories was based on a binary measure of non-smoker versus current smokers and former smokers. However, some studies have more quantitative details for smoking, such as the number of cigarettes smoked per day, age started smoking, and pack-years smoked.
Table 1
Main characteristics of all included studies
| Authors Year Location | F/U period (years) | Number of men (prostate cancer incidence) | Age (years) | Smoking categories | RR (95% CI) | Adjusted variables | (NOS) |
|---|---|---|---|---|---|---|---|
| Nilsen et al.28 2000 Europe | 12 | 22895 (644) | ≥40 | Never smoker Former smoker Current smoker | (Ref.) 0.98 (0.80–1.19) 0.96 (0.78–1.19) | Age | 7 |
| Lotufo et al.22 2000 United States | 12.5 | 21985 (996) | 40–84 | Never smoker Former smoker Current smoker: <20 cigs/d ≥20 cigs/d | (Ref.) 1.11 (0.98–1.28) 1.04 (0.73–1.48) 1.07 (0.82–1.41) | Age, BMI, height, PI, alcohol consumption, other | 6 |
| Putnam et al.24 2000 United States | 6 | 1572 (101) | ≥40 | Never smoker Former smoker Current smoker: <20 cigs/d ≥20 cigs/d | (Ref.) 1.4 (0.90–2.3) 1.3 (0.60–2.8) 1.6 (0.70–3.9) | Age | 6 |
| Rohrmann et al.11 2007 United States | 15–19 | 26810 (147) | ≥18 | Never smoker Former smoker Current smoker ≥20 cigs/d | (Ref.) 1.33 (0.85–2.10) 1.00 (0.63–1.59) 1.38 (0.75–2.54) | Age | 5 |
| 28292 (351) | Never smoker Former smoker Current smoker ≥20 cigs/d | (Ref.) 1.04 (0.80–1.36) 0.98 (0.73–1.33) 1.01 (0.64–1.57) | |||||
| Giovannucci et al.23 2007 United States | 16 | 51529 (3544) | 40–75 | Never smoker Current smoker | (Ref.) 0.98 (0.89–1.07) | Age, BMI, vigorous activity, family history of prostate cancer, other | 7 |
| Butler et al.21 2009 Singapore | 10.4 | 27293 (250) | 45–74 | Never smoker Former smoker Current smoker | (Ref.) 1.06 (0.78–1.44) 0.88 (0.65–1.19) | Age, education, vitamin D, other | 9 |
| Watters et al.18 2009 United States | NR | 283112 (16640) | 50–71 | Never smoker Former smoker Current smoker | (Ref.) 0.90 (0.87–0.93) 0.85(0.80–0.90) | Age, race, education, marital status, PSA screening test, BMI, other | 6 |
| Rohrmann et al.19 2012 Europe | Median 11.9 | 145112 (4623) | 35–70 | Never smoker Former smoker Current smoker | (Ref.) 0.96 (0.90–1.03) 0.90 (0.83–0.97) | Height, weight, education, marital status, and vigorous PI | 7 |
| Sawada et al.30 2013 Japan | 16 | 48218 (913) | NR | Never smoker Former smoker Current smoker: 0–20 20–40 ≥40 pack-years | (Ref.) 0.84 (0.70–0.99) 0.67 (0.49–0.91) 0.84 (0.70–1.02) 0.80 (0.65–0.99) | Age, public health center area, alcohol use, BMI, marital status, hx of diabetes, other | 8 |
| Bae et al.31 2013 Korea | 16 | 14450 (87) | 40–59 | Never smoker Former smoker Current smoker | (Ref.) 0.60 (0.34–1.06) 0.70 (0.43–1.13) | Age | 6 |
| Everatt et al.29 2014 Europe | 30 | 6976 (336) | 40–59 | Never smoker Former smoker Current smoker | (Ref.) 0.97 (0.74–1.26) 0.76 (0.59–1.00) | Age, education, alcohol use, and BMI | 8 |
| Park et al.17 2015 United States | Mean 13.9 | 75216 (7115) | 45–75 | Never smoker Former smoker ≥20 cigs/d Current smoker ≥20 cigs/d | (Ref.) 0.84 (0.78–0.91) 0.72 (0.63–0.83) | Age, race/ethnicity, and family history for prostate cancer | 7 |
| Perez-Cornago et al.16 2017 UK | 5.6 | 219355 (4575) | 40–69 | Never smoker Former smoker Current smoker | (Ref.) 0.93 (0.88–0.99) 0.85 (0.77–0.95) | Age, ethnicity, lives with wife or partner, BMI, physical activity, diabetes, family history of prostate cancer, other | 6 |
| Kim et al.15 2018 Korea | 8 | 1179172 (3593) | NR | Never smoker Former smoker Current smoker | (Ref.) 0.94 (0.85–1.05) 0.77 (0.71–0.84) | Age, BMI, family history of cancer, alcohol consumption, other | 6 |
| Elwood et al.27 2018 UK | Median 5.1 | 169715 (3220) | 40–69 | Current smoker Non-smoker | (Ref.) 1.11 (0.98–1.26) | Age, Townsend deprivation score, and height | 4 |
| Jacob et al.26 2018 UK | 30 | 205936 (N/A) | 18–70 | Non-smoker Smoker | (Ref.) 0.71 (0.65–0.76) | Age, BMI, other | 8 |
| Viner et al.25 2019 Canada | Mean 12.3 | 10026 (401) | 35–69 | Never smoker Former smoker Current smoker | (Ref.) 0.85 (0.69–1.06) 0.70 (0.51–0.98) | Age, marital status, education, total household income, alcohol use, other | 7 |
Five cohorts contained more details and statistical analysis for the number of cigarettes smoked per day in a current smoker and the risk of prostate cancer incidence compared to a non-smoker11,17,22,24,30. Three studies showed a minor yet insignificant increased risk of prostate cancer in current smokers who smoke ≥20 cigarettes per day11,22,24. In contrast, the remaining two studies showed a significant inverse association between heavy smoking and the incidence of prostate cancer17,30.
Five studies adjusted age as a confounder in the association between cigarette smoking and prostate cancer11,24,27,28,31. In contrast, the remaining twelve studies adjusted for several confounding variables such as body mass index (BMI), height, education level, family history of prostate cancer, prostate-specific antigen test, race, marital status, medication use, and other possible confounders. The last column shows the quality score for each article included in the systematic review and meta-analysis using the Newcastle Ottawa quality assessment scale for cohort studies (NOS). The vast majority of the studies scored high quality, while the rest scored moderate quality. However, one study had a possible risk of poor quality. Consequently, the study with the risk of poor quality was removed from the meta-analysis.
Smoking and prostate cancer incidence
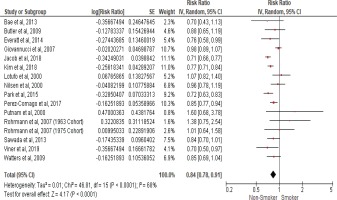
We calculated the pooled relative risk using a random effect model due to the significant heterogeny observed with I2 statistic (68%) and (Cochran Q) p<0.0001. The pooled estimates from fifteen cohort studies that assessed the risk of prostate cancer incidence in current smokers compared to non-smokers showed an inverse association with an RR of 0.84 (95% CI: 0.78–0.91), as shown in the forest plot (Figure 2), indicating that cigarette smoking is inversely associated with prostate cancer incidence.
Smoking and prostate cancer mortality
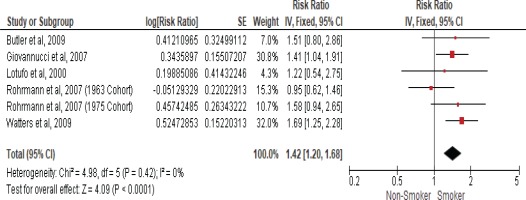
Five studies examined the association between current smokers and the risk of death from prostate cancer (Table 2). Therefore, another meta-analysis of these cohort studies was performed and showed that current smokers had a 42% higher risk of death from prostate cancer when compared to non-smokers with an RR of 1.42 (95% CI: 1.20–1.68) using a fixed-effect model due to insignificant heterogeneity with the I2 statistic (0%) and (Cochran Q) p<0.42 (Figure 3).
Table 2
Main characteristics of studies that measured mortality from prostate cancer
| Authors Year Location | F/U period (years) | Number of men (mortality from prostate cancer) | Age (years) | Smoking categorization | RR (95% CI) | Adjusted variables | NOS score |
|---|---|---|---|---|---|---|---|
| Butler et al.21 2009 Singapore | 10.4 | 27293 (47) | 45–74 | Never smoker Recent former or current smoker | (Ref.) 1.51 (0.80–2.86) | Age, education, vitamin D, other | 9 |
| Giovannucci et al.23 2007 United States | 16 | 51529 (312) | 40–75 | Never smoker Current or past smoker | (Ref.) 1.41 (1.04–1.91) | Age, BMI, vigorous activity, family history of prostate cancer, other | 7 |
| Lotufo et al.22 2000 United States | 12.5 | 21985 (113) | 40–84 | Never smoker Former smoker Current smoker: <20 cigs/d ≥20 cigs/d | (Ref.) 1.30 (0.87–1.95) 1.25 (0.45–3.49) 1.22 (0.54–2.74) | Age, BMI, height, PI, alcohol consumption, other | 6 |
| Rohrmann et al.11 2007 United States | 37 years | 26810 (240) | ≥18 | Never smoker Former smoker Current smoker ≥20 cigs/d | (Ref.) 1.01 (0.70–1.46) 0.93 (0.67–1.29) 0.95 (0.62–1.47) | Age | 5 |
| 25 years | 28292 (185) | Never smoker Former smoker Current smoker ≥20 cigs/d | (Ref.) 1.02 (0.69–1.50) 1.25 (0.84–1.87) 1.58 (0.94–2.64) | ||||
| Watters et al.18 2009 United States | NR | 283112 (394) | 50–71 | Never smoker Former smoker Current smoker | (Ref.) 1.03 (0.83–1.27) 1.69 (1.25–2.27) | Age, race, education, marital status, PSA screening test, BMI, other | 6 |
Publication bias
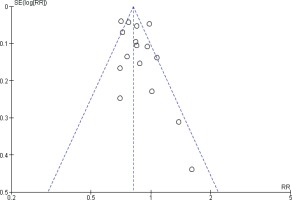
Funnel plots were performed to assess the potential risk of publication bias. For prostate cancer incidence meta-analysis, the shape of the funnel plots might indicate a slightly asymmetrical pattern; however, this asymmetry shows two small studies as a risk factor, and our result showed inverse association, so the potential risk of publication bias is unlikely affecting our result (Figure 4).
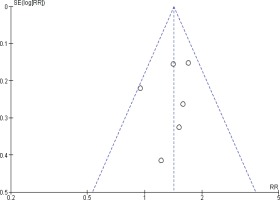
Another funnel plot was performed to assess the risk of publication bias for prostate cancer mortality meta-analysis, the shape of the funnel plot showed no clear asymmetrical pattern, so the risk of publication bias is minimized (Figure 5).
Statistical analysis results
A statistical analysis performed by excluding studies with (NOS) quality score equal to or below six indicating a risk of lower quality showed that smoking has inverse association with prostate cancer incidence with an RR of 0.83 (95% CI: 0.78–0.88)11,15,16,18,22,24,31. Similarly, another sensitivity analysis performed by excluding studies with a decreased follow-up time of 10 years or below confirmed the inverse association between smoking and the risk of prostate cancer with an RR of 0.81 (95% CI: 0.76–0.86)15,16,21,24. Therefore, the calculated pooled estimate in the prostate cancer incidence meta-analysis was not affected by studies with lower quality or decreased follow-up time, indicating that subgroup meta-analysis is unnecessary.
DISCUSSION
The pooled data in the present meta-analysis enrolling more than 2500000 participants, found that cigarette smoking has a significant inverse association with prostate cancer risk. However, a meta-analysis that included studies assessing prostate cancer mortality risk among smokers showed a 42% significantly higher risk. A sensitivity analysis was conducted due to significant heterogeny in the meta-analysis that assessed the association between cigarette smoking and prostate cancer incidence; however, neither lower study quality nor decreased follow-up period exclusion changed the crude summary effect, suggesting that the observed inverse association between smoking and prostate cancer incidence is robust and reliable.
Although smoking is associated with various solid tumors32, the association between cigarette smoking and prostate cancer incidence seems inverse. The multifactorial etiology of prostate cancer needs to be considered when interpreting these findings. Given that most casual factors in multifactorial disease have a fairly weak effect33.
The result of the present meta-analysis is consistent with the previous systematic review and meta-analysis and most epidemiological observational studies that assess the risk of prostate cancer in smokers compared to non-smokers10,13,21,26,30. The observed inverse association between smoking and prostate cancer incidence was modest. However, the association between cigarette smoking and increased mortality from prostate cancer was robust, suggesting that smoking is associated with worse prostate cancer outcomes.
The search about prostate cancer mortality among smokers was not done comprehensively as this article focuses on the incidence of prostate cancer, and the data about mortality were only among the studies that measured incidence. The result was added to have a broader picture of the effect of smoking on prostate cancer. Notably, the result found in our meta-analysis complies with previous epidemiological evidence showing that smokers have higher prostate cancer mortality compared to non-smokers34.
Strengths and limitations
This meta-analysis has several methodological strengths, including that two independent reviewers conducted the search, data extraction, and quality assessment following the PRISMA statement guidelines. Furthermore, our inclusion criteria for eligible study design were limited to cohort; therefore, the possibility of recall bias was minimized, and the clarity in temporality sequence between exposure and outcome was ascertained.
In addition, the association between cigarette smoking and mortality from prostate cancer showed no heterogeneity, indicating that the risk reported in different studies was significantly consistent. We also conducted various sensitivity analyses to examine the relationship between smoking and prostate cancer in different settings.
Furthermore, the funnel plots of incidence studies showed an asymmetrical pattern. Still, they were not indicative of publication bias, as small significant results showed smoking as a risk factor instead of being inversely associated with prostate cancer, as the summary estimate showed.
This study has a few limitations related to the included articles that might explain the inverse association observed; most of the limitations are methodological issues related to the design or data collection tools, particularly in the assessment of smoking which might not be done comprehensively. Most studies used a smoking assessment tool at the beginning of the study with three categorizations of the exposure: none, former and current smoker. Lacking repeated exposure assessment in all of the included studies might introduce the possibility of measurement bias. Then again, this limitation will increase the risk of non-differential misclassification as the exposure might change for smokers and non-smokers equally. After all, future studies need to evaluate the exposure longitudinally and assess the intensity, duration, and intermittence of smoking to reduce the possibility of measurement bias and increase the accuracy in estimating the changing spectrum of cigarette smoking habits.
Moreover, the results from most of the included articles were not presented comprehensively, as the data separating high-risk prostate cancer from low-risk prostate cancer were not mentioned in the retrieved studies11,15-17,24-29,31.
Additionally, none of the included studies mentioned any information or adjustment for prostate cancer screening. Ignorance of such an important confounder might lead to methodological bias in these studies. Important to realize that increasing evidence suggests smokers have lower compliance with prostate cancer screening programs and are more likely to have a high-grade disease at surgery, therefore a higher risk of metastasis, recurrence, and death35-37. This may be related to a delayed diagnosis among smokers or a stronger causal association of cigarette smoking with the prognosis of prostate cancer, which may be a plausible explanation for our findings. In either case, future studies need to consider the importance of adjusting for adherence to prostate cancer screening as a confounder.
Another possible explanation is that the participants who were categorized as non-smokers might be using other forms of smoking such as electronic cigarettes, hookah, or chewing tobacco, which are widely common forms of smoking. Therefore, lacking this information might explain the increased risk of prostate cancer in the non-smoker group as these details were not measured in the included articles. In order to avoid this limitation, future studies should exclude all forms of smoking in the non-smoker group when assessing the association between cigarette smoking and prostate cancer risk.
In summary, since prostate cancer is the second leading cause of cancer and the fifth leading cause of cancer mortality among men worldwide1, raising global awareness about the importance of increasing smoking cessation efforts may considerably reduce prostate cancer mortality.
CONCLUSIONS
Data from observational studies suggest that cigarette smoking has an inverse association with prostate cancer incidence. However, smokers have an increased risk of death from prostate cancer. It is important to realize that this lower risk for smokers might be attributed to low prostate cancer screening uptake among smokers, misclassification bias, or selection bias from the included original studies. In summary, this result indicates that prostate cancer incidence is less among smokers, while those who smoke and develop the disease will have a significantly worse prognosis and higher mortality risk.