INTRODUCTION
Systematic reviews and meta-analyses of observational studies have shown that current smokers have a higher risk of severe COVID-19 and death1-3. A systematic review of Mendelian randomization studies also showed that smoking traits (smoking initiation, smoking heaviness, lifetime smoking index) were associated with increased COVID-19 hospitalization, severity and mortality4. A potential mechanism is the upregulation of angiotensin converting enzyme-2 receptors (the adhesion site of SARS-CoV-2) in the respiratory tract due to cigarette smoke exposure5. Mask removal and the hand-to-mouth action of smoking may also predispose smokers to viral exposure6. The World Health Organization has recommended smoking cessation to reduce the burden of COVID-197.
Messages regarding the risk of smoking on COVID-19 susceptibility and severity may influence smoking and quitting behaviors. Unverified claims that smoking can protect against COVID-19 have been widely disseminated, especially during the early phase of the pandemic8. Our population-based survey in Hong Kong found that exposure to such misinformation was associated with increased tobacco use9. On the other hand, observational studies in the US and UK showed that a higher perceived risk of COVID-19 related to smoking was associated with increased cessation-related behaviors (e.g. smoking reduction, quit attempts, and intentions)10,11, also shown in our study in Hong Kong12. Several online experiments have found that exposure to messaging on smoking-related COVID-19 risk can increase smokers’ motivations to quit13,14, which may be mediated by fear arousal15. Nevertheless, our PubMed search (until March 2023) using the keywords ‘risk communication’, ‘health communication’, ‘health warning’, ‘messaging’, ‘COVID-19’, and ‘smoking’ did not identify any randomized clinical trial (RCT) testing the effectiveness of such messaging on smoking abstinence in a real-world setting.
Our previous trial showed the effectiveness of instant mobile messaging in promoting smoking cessation16. Our subsequent trial showed the feasibility of using instant mobile messaging to deliver cessation support amid the COVID-19 pandemic17, in which access to in-person cessation services might be constrained. The current study compared the effectiveness of communicating smoking-related COVID-19 risk using instant mobile messaging with generic cessation support via text messaging to increase abstinence in community smokers.
METHODS
Trial design
This parallel, 2-arm, pragmatic RCT was conducted under the Hong Kong Council on Smoking and Health Quit-to-Win Contest16,18-21 in Hong Kong, China. Recruitment took place amid the 3rd wave of the COVID-19 pandemic, with approximately 4200 confirmed cases and 100 deaths in a population of approximately 7.5 million during the period of the recruitment (13 June to 30 October 2020, Supplementary file Figure S1)22. Stringent preventive measures were implemented, including mandatory masking and the restriction of public gatherings to two people, but without a city-wide lockdown23. The compliance rate of mask-wearing was nearly 100%. The Institutional Review Board of the University of Hong Kong/Hospital Authority Hong Kong West Cluster approved the protocol. The trial has been registered with ClinicalTrials.gov. The results are reported according to the Consolidated Standards of Reporting Trials (CONSORT-EHEALTH) reporting guidelines.
Recruitment and participants
Participants were recruited from smoking hotspots in all 18 districts of Hong Kong or using online platforms. A total of 55 recruitment activities (booths in shopping malls and public areas) were organized at 47 community sites. University students and volunteers (n=110) from nongovernmental organizations attended a full-day online workshop and were trained as smoking cessation advisors. Similar to previous trials16,18-21, trained smoking cessation advisors proactively approached, screened and recruited smokers at smoking hotspots using the ‘foot-in-the-door’ approach24. Compared with the pre-pandemic period, fewer smokers were observed in outdoor smoking hotspots amid the pandemic6. Therefore, online advertisements were also used to recruit smokers, who were screened by a cessation advisor through video calls (eMethods in Supplementary file).
Eligible participants were Hong Kong residents who were aged ≥18 years, smoked at least 1 tobacco stick [cigarette or heated tobacco product (HTP)] per day or used e-cigarettes daily in the past 3 months, which was verified by a salivary cotinine level of ≥30 ng/mL, who was fluent in Cantonese, owned a smartphone with an instant messaging application installed, and had the intention to quit or reduce smoking. The exclusion criteria were participating in other smoking cessation programs or having physical or cognitive difficulties in communication. All eligible smokers willing to participate signed a written informed consent form.
Interventions
All participants received brief cessation advice at baseline guided by the AWARD (Ask, Warn, Advise, Refer and Do-it-again) model, which was modified from the 5As (Ask, Advise, Assess, Assist, Arrange) model for use in the community settings, as in our previous trials conducted under the Quit-to-Win Contests16,18-21. Smoking cessation advisors asked the smokers about their smoking history (Ask), warned them about the hazards of smoking using a health warning leaflet (Warn), advised them to quit or reduce smoking as soon as possible (Advice), encouraged them to use a cessation service (Refer), and repeated the above steps if they continued to smoke or had relapsed at telephone follow-up sessions (Do-it-again).
Participants in the control group received a generic health warning leaflet that included shocking pictures of diseases caused by smoking, such as coronary heart disease, stroke, and lung cancer (Supplementary file Figure S2). They also received regular text messages (i.e. participants not able to respond to the messages) with generic cessation advice for three months from baseline, with a tapering schedule from twice a week in the first month to once a week in the following two months (16 messages in total). The messages included advice on smoking hazards and quitting benefits, strategies for quitting and coping, psychosocial support, and encouragement to initiate a quit attempt.
Participants in the intervention group received a health warning leaflet that highlighted the smoking-related COVID-19 risk (Supplementary file Figure S3). This included emphasizing the risk of respiratory infection, impaired immunity and COVID-19 complications in smokers (1.4-fold to 2.4-fold increased risk of requiring intensive care, using ventilators, and death)25, as well as the higher risk of viral exposure because of mask removal, hand-to-mouth action of smoking, clustering of smokers, and secondhand smoke. They also received a combination of regular messages and chat-based cessation support for three months via WhatsApp, an instant messaging application. The schedule of regular messages was the same as that in the control group (16 messages in total). The contents were similar to those of the control group, except the messages on smoking hazards focused on smoking-related COVID-19 risk. These messages were adopted from tweets and Facebook posts from the WHO, Centre for Health Protection of the Hong Kong Government, and the University of Hong Kong (Supplementary file Figure S4). Similar to our previous trials on mobile instant messaging support16,17,26, the participants could respond to the messages and initiate conversations with a research nurse to receive cessation coaching in real-time during office hours (9 a.m. to 6 p.m., Monday to Friday). Behavioral change techniques (e.g. eliciting and answering questions, providing feedback on current behaviors, boosting motivation and self-efficacy)27 were used based on the needs of the participants. An experienced doctoral-level researcher monitored the coaching process and discussed it with the research nurse when necessary.
Randomization and blinding
A computer-generated randomization list was produced by an independent statistician using a randomized blocking schema (2, 4, or 6). Participants were individually assigned at random to groups with a 1:1 allocation ratio. Blinding the interventionists and participants was not possible because of the nature of the intervention. Outcome assessors and statistical analysts remained masked until the prespecified analyses were completed.
Outcomes
Data were collected by questionnaire at baseline and telephone interviews at 1, 2, 3, and 6 months after intervention initiation. The primary outcome was biochemically verified 7-day PPA at three months (end of treatment) and six months after treatment initiation, verified by salivary cotinine concentrations of <30 ng/mL or exhaled carbon monoxide concentrations of <4 ppm. Research staff visited or video-called participants who reported having abstained from smoking for at least 7 days at 3 and 6 months for a biochemical validation (eMethods in Supplementary file)28. Secondary outcomes included self-reported 7-day point-prevalence abstinence (PPA), smoking reduction defined by a reduction of at least 50% in daily cigarette consumption compared with that at baseline, and intervention engagement defined as having read the instant messages or text messages in the respective intervention condition (none/some/all).
At baseline and the follow-up sessions, perceived susceptibility (i.e. smoking increased the risk of COVID-19 infection) and severity (i.e. smoking increased the severity of COVID-19 infection) of COVID-19 infection due to smoking were assessed on a scale of 0 to 10, with higher scores indicating greater perceived susceptibility/severity. We also assessed participants’ COVID-19-related information exposure (‘Have you ever received information on the link between smoking and COVID-19?’, yes vs no). Process evaluations were conducted by assessing the perceived appropriateness of the messages (yes vs no) and their usefulness in increasing the motivation to quit and quit attempt, each on a scale of 0 (not helpful at all) to 4 (very useful). Participants in the intervention group were asked if they had engaged in online conversations with the research nurse and the reasons for not doing so. Those who did engage in online conversations rated their satisfaction on a scale of 0 (not satisfied at all) to 10 (very satisfied).
Statistical analysis
A meta-analysis of mobile health interventions showed an effect size [risk ratio (RR)] of 1.83 on biochemically verified 7-day PPA at six months29. Based on our prior studies12,19, we conservatively assumed an additional 10% relative increase in the intervention effect due to messaging on smoking-related COVID-19 risk; the anticipated biochemically verified PPA was 10.1% in the intervention group and 5.0% in the control group. With a type I error of 0.05, a power of 80% and an allocation ratio of 1:1, 421 participants were needed per group. Accounting for a retention rate of 75%, a total of 1122 participants was needed.
We used intent-to-treat analyses, and participants with missing outcomes were imputed as smokers with no change in tobacco consumption. Poisson regression models with robust variance estimators were used to calculate the RRs of the intervention effect on the primary and secondary outcomes. The associations of baseline perceived susceptibility/severity with 6-month biochemically verified PPA were examined, adjusting for sociodemographic and known determinants of quitting30. Sensitivity analyses were conducted for the abstinence outcomes. First, we used complete case analyses by excluding participants with missing outcomes. Second, we used multiple imputation by chained equations to impute missing abstinence outcomes, including study group, sex, age, education level, household income, previous quit attempts, cigarette dependence, and intention to quit. Rubin’s rule was used to pool the estimates from 50 imputed datasets31. Mixed-effect models were used to examine the differences in changes in the perceived susceptibility and severity of COVID-19 due to smoking from baseline through 6 months. All analyses were conducted using Stata V.15.1 (StataCorp, Texas, USA), with p<0.05 indicating statistical significance.
RESULTS
Participant characteristics
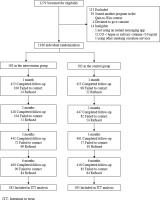
Figure 1 shows that 1166 participants were randomized to the intervention (n=583) or control group (n=583). The participants were mostly male (79.1%), aged 30–59 years (64.2%), married or cohabiting (51.4%), and had completed secondary education (62.4%); 76.4% of the participants were recruited in smoking hotspots and 23.6% via online platforms. Participants smoked an average of 13.5 cigarettes per day, 71.5% had previous quit attempts, and 50.3% were ready to quit within the next 30 days. Table 1 shows that the baseline characteristics and smoking profiles were similar between the groups. The retention rates were 71–78% at the follow-up sessions and did not differ by group (p=0.21–84).
Table 1
Baseline demographic and smoking-related characteristics (N=1166)
Smoking cessation outcomes
Table 2 shows that the primary outcome of biochemically verified PPA was not significantly different between the intervention and control groups at 3 months (9.6% vs 11.8%, RR=0.81; 95% CI: 0.58–1.13, p=0.22) or 6 months (9.3% vs 11.7%, RR=0.79; 95% CI: 0.57–1.11, p=0.18). For secondary outcomes, self-reported 7-day PPA was significantly lower in the intervention group than in the control group at 3 months (14.8% vs 21.8%, RR=0.68; 95% CI: 0.53–0.87, p=0.002) but not at 6 months (15.6% vs 19.4%, RR=0.81; 95% CI: 0.63–1.04, p=0.09). Both groups reported similar smoking reduction rates. Sensitivity analyses based on the complete case and multiple imputations showed similar results (Supplementary file Table S1). Multivariable regression showed that a higher perceived severity (RR=1.07; 95% CI: 1.00–1.13, p=0.04) but not perceived susceptibility (RR=1.01; 95% CI: 0.95–1.07, p=0.82) at baseline was associated with biochemically verified PPA at 6 months (Supplementary file Table S2).
Table 2
Primary and secondary outcomes by ITT analysis (N=1166)
| Outcomes | Intervention group (N=583) n (%) | Control group (N=583) n (%) | RR (95% CI) | p |
|---|---|---|---|---|
| Primary outcomes | ||||
| Biochemically validated 7-day PPA | ||||
| 3-month | 56 (9.6) | 69 (11.8) | 0.81 (0.58–1.13) | 0.22 |
| 6-month | 54 (9.3) | 68 (11.7) | 0.79 (0.57–1.11) | 0.18 |
| Secondary outcomes | ||||
| Self-reported 7-day PPA | ||||
| 1-month | 48 (8.2) | 73 (12.5) | 0.66 (0.47–0.93) | 0.017 |
| 2-month | 65 (11.1) | 91 (15.6) | 0.71 (0.53–0.96) | 0.026 |
| 3-month | 86 (14.8) | 127 (21.8) | 0.68 (0.53–0.87) | 0.002 |
| 6-month | 91 (15.6) | 113 (19.4) | 0.81 (0.63–1.04) | 0.09 |
| Smoking reductiona | ||||
| 1-month | 128 (22.0) | 131 (22.5) | 0.98 (0.79–1.21) | 0.83 |
| 2-month | 123 (21.1) | 128 (22.0) | 0.96 (0.77–1.20) | 0.72 |
| 3-month | 120 (20.6) | 108 (18.5) | 1.11 (0.88–1.40) | 0.38 |
| 6-month | 119 (20.4) | 102 (17.5) | 1.17 (0.92–1.48) | 0.21 |
Change in perceived susceptibility and severity
Table 3 shows that the perceived susceptibility and severity of COVID-19 infection were not significantly different between the groups at baseline and at the follow-up sessions, except perceived severity was higher in the intervention group than in the control group at 2 months (6.2 vs 5.9; p=0.045). Exploratory analyses using mixed-effect models showed no significant intervention effect on the changes in perceived susceptibility (p for group × time interaction = 0.19) but a marginally significant effect on perceived severity (p for group × time interaction = 0.08) from baseline through 6 months.
Table 3
Changes in perceived susceptibility and severitya of COVID-19 due to smoking by groups (N=1166)
Intervention engagement and ratings
Table 4 shows that the proportion of participants who had read the instant/text messages was similar between the two groups. Biochemically verified 7-day PPA at 6 months was higher in engaged subgroups (e.g. read messages, had conversations with research nurse, and read messages and had conversations) than non-engaged participants (Supplementary file Table S3). The perceived appropriateness of the messages was high overall, although it was significantly lower in the intervention group than in the control group (86.5% vs 90.6%, p=0.04). Similarly, the perceived usefulness of the messages in increasing motivation to quit (2.3 vs 2.4, p=0.02) and quit attempts (2.2 vs 2.4, p=0.02) was significantly lower in the intervention group.
Table 4
Intervention engagement and ratings (N=1166)
| Intervention group (N=583) n (%) | Control group (N=583) n (%) | p | |
|---|---|---|---|
| Intervention engagement | |||
| Have ever read instant/text messages | 0.11 | ||
| Nonea | 94 (16.1) | 113 (19.4) | |
| Some | 142 (24.4) | 116 (19.9) | |
| All | 347 (59.5) | 354 (60.7) | |
| Rating of the messages | |||
| The intensity of messages was appropriate (yes vs no) | 423 (86.5) | 426 (90.6) | 0.04 |
| The contents of messages increased motivation to quitb, mean ± SD | 2.3 ± 1.0 | 2.4 ± 1.1 | 0.02 |
| The contents of messages increased quit attempts, mean ± SD | 2.2 ± 1.0 | 2.4 ± 1.1 | 0.01 |
| Exposure to information on COVID-19 and smoking | |||
| Baseline | 192 (33.3) | 207 (36.1) | 0.32 |
| 1-month (cumulative) | 372 (64.2) | 309 (53.4) | <0.001 |
| 2-month (cumulative) | 426 (73.6) | 388 (66.7) | 0.01 |
| 3-month (cumulative) | 482 (83.2) | 448 (77.0) | 0.008 |
| 6-month (cumulative) | 489 (84.5) | 470 (80.8) | 0.09 |
In the intervention group, 49.4% (n=288) of the participants had conversations with the research nurse, with a mean satisfaction score of 8.5 (SD=1.9) on a scale of 0 to 10. Being ‘too busy’ (49.5%) was the most common reason for not conversing with the research nurse, followed by ‘don't want to talk about cessation-related topics online’ (32.9%), ‘not interested’ (19.7%), and ‘not useful’ (5.4%).
DISCUSSION
This is the first RCT testing the effectiveness of messaging on smoking-related COVID-19 risk in increasing smoking abstinence in a real-world setting. Contrary to our expectations, communicating smoking-related COVID-19 risk via instant mobile messaging did not increase biochemically verified 7-day PPA at 3 and 6 months when compared with generic cessation support via text messaging amid the COVID-19 pandemic. However, the 6-month verified PPA (approximately 10%) in both groups was much higher than that of the intervention groups in our previous RCTs conducted under the Quit-to-Win contests before the COVID-19 pandemic (ranging from 5.6% to 9.0%)16,18-21. A 2019 Cochrane review of 13 RCTs showed that text messaging-based behavioral support was effective in promoting abstinence29. Our findings indicate that communicating messages on smoking-related COVID-19 risk with a research nurse via instant messaging did not confer additional benefits.
The intervention was designed to promote cessation by increasing the perceived smoking-related risk of COVID-19, which has been found to be associated with intermediate cessation outcomes such as the motivation to quit in observational and experimental studies10-14. Our secondary analyses also showed that greater perceived severity of COVID-19 due to smoking was associated with 6-month verified PPA, adjusting for known determinants of successful cessation. However, we did not find a significant difference in changes in perceptions between the two groups, which may explain the null effect on abstinence. Subgroup analyses between online and face-to-face recruitment found no significant difference in abstinence, suggesting the similar null effects of interventions in both groups.
Several reasons may underlie the failure of the intervention to change smoking-related COVID-19 risk perception. Our survey conducted prior to the trial showed that most smokers did not think smoking increased COVID-19 susceptibility (72%) or severity (58%)12. However, our trial participants showed relatively high levels of perceived susceptibility and severity (mean scores = 7.7 and 6.2 out of 10, respectively) at baseline. This ‘ceiling effect’ might have constrained the room for increasing the risk perception level. Second, over 80% of the participants in both groups reported exposure to information on COVID-19 and smoking from any sources during the study period. The control group likely was exposed to such information from other sources, such as publicity by the local health authority and cessation services. Such contamination might have biased the intervention effect toward the null. Finally, participants’ ratings for the messages were significantly lower in the intervention group than in the control group, which suggests that messaging on smoking-related COVID-19 risk was less preferred compared to generic messages and cessation support. Prior qualitative studies on mobile messaging showed that smokers might not be receptive to messages that repeatedly emphasize the hazards of smoking (loss-framed messages)32,33. Further research is warranted to determine the optimal ‘dose’ to communicate smoking-related COVID-19 risk without demotivating smokers.
Studies on smokers’ affective responses to messages on smoking and COVID-19 also provide some insights into our null findings. An experiment showed that messages on smoking-related COVID-19 risk (vs traditional risk) elicited similar levels of reactions (e.g. attention, negative affect) and were perceived to be similarly effective in discouraging smoking14. Likewise, another experiment showed no difference in valence or arousal elicited by COVID-19 or traditional graphic health warnings on cigarette packaging34. The experiment also showed that smokers with lower delay discounting (i.e. the preference for smaller immediate rewards over larger but delayed rewards) were more likely to be aroused by COVID-19 warnings34. The much milder impact of COVID-19 in Hong Kong than in most other places worldwide might have led to a lower perceived benefit of cessation in reducing COVID-19 risk and, thus, the abstinence rate.
Strengths and limitations
The strengths of the study include the randomized trial design conducted with a large cohort of smokers (n=1166) in a real-world setting with the use of an active comparison group. Another strength is the use of biochemically verified PPA as the outcome with a satisfactory participation rate (64%; 144/226) despite the difficulties of conducting in-person validation amid the pandemic.
This trial also had several limitations. First, despite a satisfactory retention rate of 71% at 6 months, given the minimal contact between the participants and researchers, non-response bias could not be excluded. However, our sensitivity analyses based on multiple imputations and complete cases yielded similar results to those of the main analyses. Second, as discussed, most participants in the control group were exposed to information on COVID-19-related smoking risks, which might reduce the intervention effect size, if any. However, given the real-world trial design, such contamination is difficult to control35. Third, the secondary outcome of intervention engagement (i.e. having read the instant messages or text messages) was based on self-report. Finally, our trial was conducted in Hong Kong, where the prevalence of smoking is low (9.5%), and smokers are predominantly male36. The generalizability of the findings to other places is unclear.