INTRODUCTION
Objective smoking measures are useful to verify self-reported smoking status by individuals participating in harm reduction or smoking cessation interventions and studies1. Exhaled carbon monoxide (eCO) is widely used for these purposes because it is less expensive and less invasive than other measures, such as serum or urinary cotinine, and provides immediate results that assist in motivating patients to quit1,2. The piCO+ Smokerlyzer® is commonly used to measure eCO levels in clinical and research settings3 because of its high validity and reproducibility in discriminating smokers from non-smokers4-6.
To date, the validity and reproducibility of the iCOTM Smokerlyzer®7, an eCO measuring device designed for use with a smartphone and marketed primarily as a self-monitoring tool, is unknown. If found to perform as well as the piCO+ Smokerlyzer®, despite being intended for single-patient use, the iCOTM Smokerlyzer® could potentially be a research tool in smoking intervention studies, and at a lower cost. In clinical settings, the iCOTM Smokerlyzer® may also be useful where limited devices are available and where frequent monitoring may support therapeutic goals such as harm reduction for individuals on methadone-maintenance therapy (MMT)8,9.
This exploratory study aimed to compare the validity and reproducibility of the iCOTM Smokerlyzer® with those of the piCO+ Smokerlyzer®, and correlate eCO levels with an established measure of nicotine dependence.
METHODS
Study design
Participants from three methadone clinics (University Malaya Medical Center, San Peng, and Chow Kit) in Kuala Lumpur, Malaysia were recruited from December 2017 to January 2018. Participants who were aged ≥18 years, on MMT for two months or more, who had an established therapeutic compliance and were not on marijuana or any other recreational drugs (as determined by routine urinary drug tests), and smoked at least one cigarette daily for the past one month, were approached for consent to take part in the study. Participants who could not understand the device instructions or who were medically unstable were excluded. The Medical Ethical Committee of University Malaya Medical Center approved the study protocol (MECID: 20146-331).
Procedure
Sociodemographic data were obtained and the Malay version of the Fagerström Test for Nicotine Dependence (FTND-M) was administered10. This instrument has moderate validity in distinguishing smokers with nicotine dependence from their non-nicotine-dependent counterparts with a cut-off of 2, and positively correlates with piCO+ Smokerlyzer®-measured eCO levels10,11.
The tobacco Q-score12 was calculated by dividing by two the total number of cigarettes consumed on the two days before the day of the study, enabling each participant to be categorized as light (≤5 cigarettes/ day), moderate (6–19 cigarettes/day) or heavy smoker (≥20 cigarettes/day)13,14.
Both devices were sanitized with anti-bacterial cleaning wipes between participants as per manufacturer recommendations3,7, using makeshift single-use mouthpieces for both devices to reduce transmission of fluids between participants; the smartphone Smokerlyzer application was used with the iCOTM Smokerlyzer®. The instructions for both devices were: to completely exhale, take a deep breath, hold the breath for 15 s, and exhale completely and slowly into each device, which yielded values in parts per million (ppm). Each participant provided four samples with 5-minute intervals between samples, beginning with the piCO+ Smokerlyzer®, and alternating with the iCOTM Smokerlyzer®.
Data analysis
Descriptive statistics were used to examine characteristics data. The piCO+ Smokerlyzer® readings were converted into ordinal categories (0–6; 7–10; 11–15; 16–20; 21–25; 26–30; and ≥31 ppm) to be compared with the ordinal categories in the initial results interface obtained from the iCOTM Smokerlyzer®; category readings from both devices were averaged for further analyses.
Partial correlations adjusting for covariates of age, tobacco Q-score and FTND-M scores, which were found to significantly influence the correlation between piCO+ and iCOTM Smokerlyzer® readings, were performed using SPSS v25. Additionally, correlations between first and second piCO+ and iCOTM Smokerlyzer® readings and between the iCOTM Smokerlyzer® readings and FTND-M scores were also performed.
RESULTS
The participants’ mean age was 47.9 years (Table 1). The average daily methadone dose was 69.7 mg, the mean tobacco Q-score was 12.3 cigarettes/day and the mean FTND-M score was 3.9. Most participants were male (92.5%), Malay (73.3%) and moderate smokers (55.5%).
Table 1
Baseline sociodemographic characteristics of study subjects (N=146 )
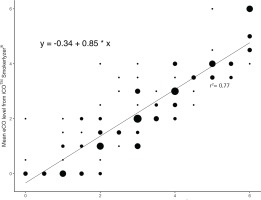
Mean eCO levels were significantly correlated between both devices (r=0.86, p<0.001; Figure 1), after adjusting for covariates of age, tobacco Q-score and FTND-M score. First and second device readings were significantly correlated with each other, after controlling for the same covariates (r=0.94 for categorical values of the iCOTM Smokerlyzer®, p<0.001; r=0.91 for integer values of the piCO+ Smokerlyzer®, p<0.001; r=0.86 for categorical values of the piCO+ Smokerlyzer®, p<0.001). Post hoc analyses using the Bonferroni procedure revealed that iCOTM Smokerlyzer® readings were significantly lower than the corresponding piCO+ Smokerlyzer® readings by 0.82 (95% CI: 0.69–0.94, p<0.001), and subsequent linear regression analyses confirmed a significant intercept of -0.34 (95% CI: -0.61 – -0.07, p=0.016). Mean eCO levels of the iCOTM Smokerlyzer® positively correlated with FTND-M scores (r=0.22, p<0.01).
DISCUSSION
In this exploratory study, the significant correlation found between the readings of the iCOTM Smokerlyzer® and the piCO+ Smokerlyzer®, indicates that the iCOTM Smokerlyzer® may have validity equivalent to the piCO+ Smokerlyzer® device that has been shown to be highly valid in discriminating between smokers and non-smokers4-6. With regard to reproducibility of the iCOTM Smokerlyzer®, both readings were significantly correlated (r=0.94). Interestingly, the corresponding piCO+ Smokerlyzer® value was lower (r=0.91 for raw values, r=0.86 when in grouped categories, p<0.001 for all three correlations), suggesting that the iCOTM Smokerlyzer® performed very well in terms of reproducibility when grouped into categories.
Additionally, the post hoc analysis finding and subsequent regression analyses with a significant intercept (Figure 1) suggest that one or more of the iCOTM Smokerlyzer® devices may be consistently yielding underestimates of eCO levels. Therefore, further guidelines are needed to recognize and rectify this calibration error, indicating that routine checks are needed against another calibrated device such as the piCO+ Smokerlyzer®. Finally, the finding that eCO levels of the iCOTM Smokerlyzer® correlated positively with the FTND-M scores warrants further research on whether reducing smoking in people with high nicotine dependence is a step towards smoking cessation.
This study did not assess raw values of the iCOTM Smokerlyzer® or abstinence cut-off points, thus restricting further analyses, such as Bland-Altman analysis, and the potential as a clinical utility. A study by Karelitz et al.15 had demonstrated lack of agreement and differences of 1.5–6.0 ppm between both monitors, thereby suggesting a lack of interchangeability between readings of both monitors. Additionally, the iCOTM Smokerlyzer® has a cited accuracy of 15% for each 1 ppm7, compared to <3% for the piCO+ Smokerlyzer®3. Consequently, the use of broad categories may indeed be supported, aiming to reduce the differences between devices, and in the process potentially increase interchangeability between readings of both monitors, which would need to be confirmed through further research.
Nevertheless, assessing the iCOTM Smokerlyzer® against the piCO+ Smokerlyzer® has yielded some results of clinical significance. The piCO+ Smokerlyzer® has been extensively studied and shown to be reproducible and reasonably accurate in determining patients who are abstinent, based on objective scores4-6. This study demonstrates that the categories obtained from the iCOTM Smokerlyzer® correlate with the categorical grouped integers shown on the piCO+ Smokerlyzer®. As such, there is a possibility for the more economical and user-friendly iCOTM Smokerlyzer® to be researched in greater detail in order to assess its suitability for use in research and clinical environments.
CONCLUSIONS
The iCOTM Smokerlyzer® yielded highly reproducible results that are potentially comparable to the piCO+ Smokerlyzer®, pending further calibration guidelines. As a more economical and user-friendly device than the piCO+ Smokerlyzer®, the iCOTM Smokerlyzer® therefore has potential use in smoking cessation studies. Additionally, many individuals could benefit from using the iCOTM Smokerlyzer®, to assess the true extent of their cigarette consumption, such that behavioral and pharmacological smoking interventions may be studied and implemented in a timely manner.